Chapter 89 Isolated hepatic perfusion for extensive liver cancer
Overview
Each year, isolated hepatic metastases from a variety of primary malignancies pose a significant clinical dilemma for tens of thousands of patients. For a small percentage of patients, surgical resection or ablation is effective in controlling clinically apparent disease, but for many patients with colorectal, gastrointestinal, or neuroendocrine tumors or ocular melanoma, the number of tumors and volume of affected liver render resection and/or ablation incapable of meaningful disease control (see Chapter 81A, Chapter 81B, Chapter 81C ). In more selected circumstances, metastases arising from tumors of the breast, skin, and soft tissue can be present solely as hepatic disease, with patient quality of life and survival dictated by the ability to control liver-based disease.
Based on its unique vascular anatomy (see Chapter 1B), the liver is a favorable site for delivery of regional therapy (see Chapter 86), as complete control of circulatory inflow and outflow can be readily obtained. Additionally, established tumors in liver derive the majority of blood flow from the arterial tree, and the portal vein is maintained as the primary source of nutrient flow to the hepatic parenchyma, allowing intraarterial delivery to effectively concentrate drug within tumor-bearing areas of the liver. Animal models demonstrate that nearly 100% of blood delivered to tumors arises from the arterial circulation versus 25% for normal liver (Breedis & Young, 1954; Ridge et al, 1986). The ability to obtain complete vascular isolation also permits the delivery of clinically relevant levels of hyperthermic and/or biologic agents that would otherwise be too toxic or technically impractical to deliver. For patients with multiple hepatic metastases, the likelihood of additional subclinical disease being present within the liver increases with greater tumor volume, and thus the ability to treat the entire diseased organ through regional perfusion strategies allows the targeting of micrometastatic disease within the diseased organ.
The initial report detailing the clinical use of IHP was published in 1961 by Robert Ausman from the Roswell Park Cancer Center, describing his experience with both a porcine treatment model along with five treated patients (Ausman, 1961). In this brief report, evidence of antitumor efficacy was seen in two patients after a 60-minute perfusion with melphalan. Over the ensuing 2 decades, additional small series examined the utility of prolonged hyperthermic perfusions (Skibba & Quebbeman, 1986) without drug, as well as normothermic perfusions utilizing melphalan (Hafström et al, 1990) or mitomycin-C and 5-fluorouracil (5-FU) (Aigner et al, 1988), and were described by Skibba (Medical College of Wisconsin) and multiple European groups, respectively. The absence of long-term follow-up and the presence of significant toxicity prevented widespread adoption of this approach, until interest in the field of regional therapy was reignited by Leinhard and colleagues’ 1992 publication that detailed the successful delivery of a combination of melphalan, tumor necrosis factor (TNF), and interferon-α via hyperthermic isolated limb perfusion in a group of 29 patients with advanced extremity sarcoma or melanoma. Clinical results demonstrated a 90% complete response rate for patients with in-transit melanoma along with an 80% limb salvage rate for patients with advanced sarcoma. Of equal or greater significance was the demonstration that meticulous surgical technique could result in very effective vascular isolation with a resultant decrease in out-of-field drug exposure and associated toxicity. When this increased attention to circuit integrity and leak monitoring was applied to patients undergoing IHP, a similar decrease in systemic toxicity permitted more widespread investigation of this clinical approach.
Within the United States, a significant effort in the development and refinement of vascular isolation-perfusion techniques was initiated by Drs. Douglas Fraker and Richard Alexander within the Surgery Branch of the National Cancer Institute (NCI) (Alexander et al, 1998, 1999).
Surgical Technique
Preoperative evaluation of patients thought suitable for IHP should include an assessment of the patients’ overall cardiovascular risk factors, the extent of both intrahepatic and extrahepatic tumor malignancy, and an assessment of the liver functional status (see Chapter 2). Standard preoperative cardiac clearance should include a treadmill stress test, as the induction of venovenous stress can lead to the induction of atrial fibrillation in patients so disposed. Hepatic reserve is important to assess, as the dose-limiting toxicity observed in Phase I trials was liver based, and it was seen in patients with more than 50% of hepatic replacement with tumor or a serum bilirubin level higher than 3 mg/dL. For patients with colorectal cancer and a significant chemotherapy history that includes either oxaliplatin and/or irinotecan, a biopsy assessment of the uninvolved hepatic parenchyma must be done to rule out extensive portal inflammation, hepatic congestion, and steatohepatitis (see Chapter 65). Patients who have had extensive portal of hepatic venous dissection associated with major hepatectomy should be approached with caution.
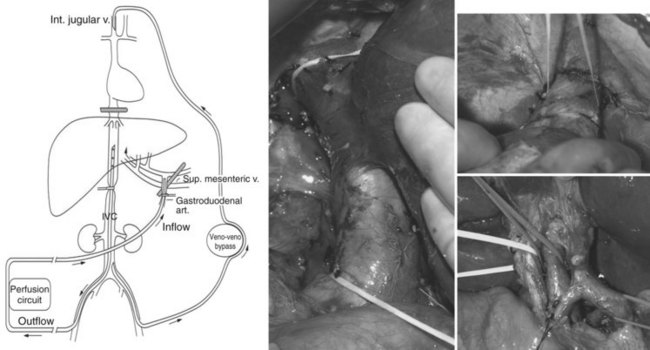
Preparation of the liver for perfusion includes completion of a cholecystectomy and full mobilization the liver (Alexander, 2005; Libutti et al, 2000). All lateral attachments are taken down so that the vena cava is fully visualized, and all retroperitoneal venous tributaries are taken down to ensure there will be no leak of chemotherapy from the isolated segment of the retrohepatic inferior vena cava (IVC). The duodenum is mobilized, and the IVC is mobilized from the renal veins to the hepatic veins. The right adrenal vein is suture ligated and divided, but both phrenic veins are preserved. The hepatic artery is identified, and the gastroduodenal artery (GDA) is mobilized from its origin for a length of 2 cm to serve as the arterial inflow catheterization site. Nodal tissue in the porta hepatis is dissected to allow clamping of the portal structures, with the hepatic artery clamp proximal to the takeoff of the GDA. Minor accessory right and left hepatic vessels may be ligated, but replaced or accessory arteries of a significant size are prepared for cannulation along with the GDA.
After completion of dissection, the patient is heparinized to an activated clotting time longer than 400 seconds. An external venovenous bypass circuit is established by placing a cannula into the left femoral vein and advancing it into the infrarenal IVC and then advancing a second cannula through the internal jugular vein into the superior vena cava (SVC). This allows maintenance of the systemic circulation by actively shunting IVC blood during treatment. Once the venous bypass has been established, the intrahepatic (IHP) circuit is constructed (see Fig. 89.1), and the GDA is ligated distally. The inflow perfusion cannula is positioned in the proximal GDA; once it has been secured, a cross clamp is placed across the entire porta hepatis, including the common hepatic artery, bile duct, and portal vein. The perfusion outflow cannula is inserted into the retrohepatic IVC via a percutaneous cannulation of the right femoral vein, and a tourniquet is placed around the cannula at the level of the suprarenal infrahepatic IVC. The suprahepatic IVC is then cross clamped, completing the vascular isolation of the liver, and perfusion is initiated; the perfusate consists of approximately 500 mL Ringer’s lactate to which 2 U packed red blood cells are added.
Once perfusion is initiated, flow through the isolated circuit is maintained between 400 and 600 mL/min. The routine use of leak monitors has been abandoned, as detectable leak is rare in the presence of a stable circuit reservoir (Barker et al, 1995). Temperature probes are placed into the anatomic right and left lobes of the liver, and the perfusate is heated to maintain hepatic hyperthermia of 40° C. Once hyperthermia is obtained, and perfusion parameters are stable, melphalan (1.5 mg/kg ideal body weight) is administered into the arterial limb of the isolated perfusion circuit over 5 minutes. The liver is perfused for 1 hour, after which time the circuit is flushed with 2000 mL saline and 500 mL colloid to flush all chemotherapy from the hepatic vasculature. The portal and suprahepatic IVC clamps are removed to allow native blood flow to be restored. The GDA is either suture ligated or a hepatic arterial infusion pump is placed. The venovenous bypass circuit is halted, and both the femoral vein and right internal jugular vein catheters are removed; anticoagulation is reversed with protamine and 2 U fresh frozen plasma.
Postoperative care focuses on the maintenance of normal coagulation profiles and standard fluid resuscitation (see Chapter 22). Heparin should be avoided, as high levels of heparin-induced antibodies are common (Masucci et al, 1999); this phenomenon is rare but can lead to devastating consequences, if heparin is administered in the early postoperative setting.
Pharmacokinetic analyses performed during early phase IHP trials have demonstrated that complete vascular isolation is routinely achieved with no detectable levels of melphalan in the systemic circulation (Lans et al, 2001). A small amount of systemic drug observed in the immediate postperfusion period has been addressed by unclamping the portal clamp for 10 seconds during the washout phase, facilitating clearing of the portal circulation. A transient significant elevation of aspartate and alanine transaminases was universally observed but was self-limited and resolved within 7 days. Biliary congestion and cholestatic jaundice is rare when proper patient selection is maintained with regard to underlying liver disease and tumor volume. Overall operative mortality rate is 4% across single-institution experiences (Alexander, 2005), and operative times and blood loss have decreased with the implementation of less invasive catheterization techniques, with operative times of less than 5 hours and mean operative blood loss less than 500 mL. An additional 500 mL of blood loss results from the removal of melphalan-contaminated hepatic circuit blood. Recent dose-escalation studies performed by Zeh and Bartlett at the University of Pittsburgh have demonstrated effective isolation and safety utilizing both 5-FU and oxaliplatin (Colville et al, 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree