Fig. 1.1
Roux-en-Y gastric bypass (RYGB)
In 1994, Wittgrove and Clark performed the first laparoscopic Roux-en-Y gastric bypass (LRYGB) . The proximal anastomosis was created using a circular stapler, with the introduction of the stapler anvil transorally using a peroral endoscopically placed wire similar to that used for percutaneous endoscopic gastrostomy (PEG) tube placement [25]. De la Torre and Scott described a variation of this technique with the introduction of the anvil of the stapler trans-abdominally [26]. In 1999, Higa reported LRYGB using a laparoscopic hand-sewn 2-layer gastrojejunostomy [27].
Long-term results from a number of different studies over the last three decades demonstrate that the excess weight loss with RYGB is 60–70 % at 5 years, 55–60 % at 10 years, and 50–62 % at 14 years [28, 29]. Although no longer the most commonly performed operation in many centers, the LRYGB is still considered by most bariatric surgeons to be the “ gold standard” operation against which all other procedures are measured.
1.6 Gastroplasty
Many different types of gastroplasty have been used over the past three decades for weight loss purposes. However, these techniques have gradually fallen out of favor given their high rate of complications and the frequently inadequate weight loss obtained. The first gastroplasty procedure was performed by Mason and Printen in 1971 [30]. In their technique as originally described, they incompletely divided the stomach horizontally from the lesser curvature to the greater curvature, leaving a small conduit for the physiologic passage of food contents distally. This technique was ultimately unsuccessful in accomplishing adequate long-term weight loss and was abandoned [31]. In 1980, after several modifications of the technique, Mason described the vertical-banded gastroplasty or VBG [32]. In this technique, the stomach was stapled vertically but not divided using a TA-90 stapler after creating a through-and-through window across the anterior and posterior stomach walls. The remaining stomach conduit next to the lesser curvature was then banded with a 1.5 cm wide polypropylene mesh collar creating a small 30 ml gastric pouch. A modification to this technique was also described by Laws, who used a silicon ring in place of the polypropylene mesh as a permanent, nonexpendable restriction of the pouch outlet [33]. Another modification of Mason’s technique was described in 1990 by MacLean, in which the vertical staple line was created using a cutting stapler, thereby completely separating the stomach pouch from the greater curvature [34]. In 1994, Hess and Hess performed the first laparoscopic vertical-banded gastroplasty [35].
Because of significant food restriction and weight loss with the VBG technique, during the 80s and beginning of the 90s, it was used in many centers as the first line of treatment for morbid obesity. However, rates of weight regain were then noticed to be high due to patient’s adaptation to high calorie food intake [34, 36, 37]. Complications of the VBG included gastric outlet obstruction secondary to stricture formation, perforation and leak, gastroesophageal reflux and staple line dehiscence with recanalization of the gastric lumen among the most common [38, 39]. For these reasons, the approach was ultimately abandoned.
1.7 Current Bariatric Procedures
1.7.1 Laparoscopic Gastric Bypass
The loop gastrojejunostomy, introduced by Mason and Ito in 1967, gradually evolved into the Roux-en-Y gastric bypass described by Griffen in 1977, with the advantage of reduced bile reflux and marginal ulcers and lowered anastomotic tension [40] (Fig. 1.1). The first series of LRYGB was reported in 1994 by Wittgrove and Clark, with an end-to-end gastrojejunostomy created using an endoscopically introduced anvil [25]. In the LRYGB, the uppermost part of the stomach is partitioned using a cutting surgical stapler to create a small gastric pouch, typically < 30 ml in size. A Roux limb, usually 100–150 cm long, is brought up to the stomach pouch and anastomosed using sutures, staples or a combination of the two. The Roux limb can be brought up in front of the colon (antecolic) or behind the colon (retrocolic). Superiorly, the Roux limb can travel in front of the bypassed stomach (antegastric) or behind it (retrogastric). Gastric juices from the bypassed stomach mix with bile from the liver and pancreatic secretions and pass through approximately 40–100 cm of jejunum referred to as the biliopancreatic limb, before joining the Roux limb to form the “common channel.” Internal hernia spaces behind the Roux limb (Petersen defect) and at the distal anastomosis are closed to prevent future bowel entrapment; in retrocolic bypasses, the Roux limb is sutured to the retrocolic tunnel as well [41].
Multiple studies have confirmed that LRYGB is effective at achieving weight loss and resolving comorbidities, while maintaining an acceptably low rate of complications. A systematic review and meta-analysis from 2014 reported the average excess weight loss (EWL) after gastric bypass to be 64–73 % 2–3 years after surgery among 31 randomized controlled trials (RCTs) and 51–78 % among 8 observational studies [42]. Fewer studies were found reporting EWL at 5 years; the average EWL at 5 years among two observational studies was 58 %.
LRYGB is one of the most effective operations for achieving remission of T2DM. Since Pories et al. first described the effect of RYGB on the remission of obesity and diabetes mellitus [43], a large body of supporting literature has accumulated [3, 4, 44, 45]. In a meta-analysis of 621 studies, the average rate of diabetes resolution was 80.3 % among patients undergoing gastric bypass [3]. Four randomized controlled trials have reported that subjects who underwent RYGB were found to have a significantly higher rate of diabetes remission than patients who were only treated with medical therapy [46–49]. The same studies have also shown significant difference in secondary endpoints including hypertension, dyslipidemia, and proteinuria in patients undergoing surgery versus those being treated medically [48, 50]. RYGB has also shown to be effective in inducing diabetes remission in patients with a BMI ≤ 35 kg/m2 [51].
Initially, gastric bypass was accompanied by a relatively high mortality rate. With refinements in technique through experience and proper patient selection, mortality has significantly decreased over time. A systematic review and meta-analysis from 2014 reported a perioperative mortality rate of 0.08 % for RCTs and 0.38 % for observational studies [1]. The mortality rate for greater than 30 days was reported to be 0.39 for RCTs and 0.72 for observational studies. The rate of complications after LRYGB was found to be 21 % and 12 % among RCTs and observational studies, respectively. Reoperation rates were approximately 2.6 % and 5.3 % among RCTs and observations studies.
Complications are typically classified as early or late based on their occurrence before or after the 30-day mark. The most common early complications include leakage, stenosis and bleeding. Most leaks occur at the gastrojejunostomy, though leaks can also infrequently be seen at the entero-enteral anastomosis and at the gastric pouch. Late complications include stricture formation at the gastrojejunal anastomosis, which may be the result of tension or ischemia at the anastomosis, subclinical leaks and/or exposure to excessive gastric acid [52]. Internal hernias, which may occur within the Petersen defect or at the enteroenterostomy site, can be catastrophic, and should be closed at the time of the initial operation [41]. Marginal ulceration, which may be a result of gastro-gastric fistula, is also seen infrequently and can typically be managed nonoperatively. The incidence of complications has been found to decrease with increasing experience of the surgical team over time [53].
1.7.2 Laparoscopic Sleeve Gastrectomy
The sleeve gastrectomy was first described in 1988 by Hess and contemporaneously by Marceau as a component of their biliopancreatic diversion with duodenal switch, or BPD-DS. This operation was a modification of the biliopancreatic diversion (BPD) operation first performed by Scopinaro in Italy [54, 55]. The BPD-DS involved creating a vertical gastric pouch approximately 100–150 ml in volume by resecting the greater curvature and preserving the antrum and pylorus (Fig. 1.2). The duodenum was divided at its first portion and the proximal aspect anastomosed to the ileum, creating the alimentary channel. This anatomy provided a significantly decreased rate of marginal ulceration, intestinal perforation, hypoproteinemia, hypocalcemia, and dumping syndrome, with maintenance of excellent weight loss, when compared to the original BPD [56].
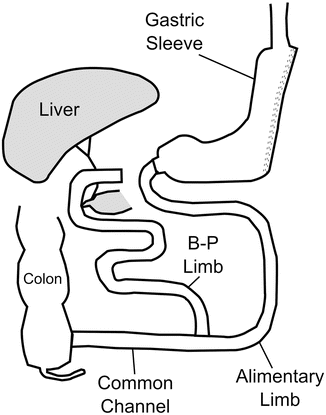

Fig. 1.2
Biliopancreatic diversion with duodenal switch (BPD-DS)
The first laparoscopic BPD-DS was performed by Gagner in New York in 1999 [57]. In an attempt several years later to decrease the morbidity and mortality of patients with a BMI over 60 undergoing BPD-DS, operations on these high-risk patients were performed in two stages: the technically simpler laparoscopic sleeve gastrectomy to allow for initial weight loss and comorbidity resolution, followed by completion of the BPD-DS anatomy approximately 6–12 months later [58–60]. Many of these staged patients achieved substantial weight loss with the sleeve gastrectomy alone, which ultimately led to its recognition as a stand-alone primary weight loss operation [61, 62]. Over time, laparoscopic sleeve gastrectomy (LSG), also referred to as vertical sleeve gastrectomy has gained increased support from both surgeons and patients.
In the LSG, the greater curvature of the stomach is mobilized by dividing the gastrocolic omentum up to the angle of His superiorly and inferiorly to the antrum approximately 3–6 cm proximal to the pylorus [63]. The linear endoscopic stapler is serially applied to the stomach, beginning approximately 3–6 cm proximal to the pylorus and continuing upward. Staples designed for thicker tissue (e.g., black cartridge) are commonly used for the antral division, while reloads designed for thinner tissue (e.g., purple or blue) may result in better tissue compression and decreased bleeding. Staplers are fired adjacent to a calibrating bougie , typically 32–46 Fr size, which prevents excessive narrowing of the tubularized stomach (Fig. 1.3). Many surgeons feel that the use of buttressing material or oversewing of the staple line may potentially decrease the rate of postoperative bleeding or leakage, although the data are equivocal. This author’s group has used both imbrication of part or all of the staple line with a running 2–0 polydioxanone suture and staple line buttressing with excellent results. The excised portion of the stomach is then removed through the largest port site, with or without wound protection. An intraoperative esophagogastroduodenoscopy may be performed to assess the patency of the sleeve, ensure intraluminal hemostasis, and rule out leakage from the staple line. Alternatively, a leak test may be performed with instillation of air or methylene blue dye through an orogastric tube.
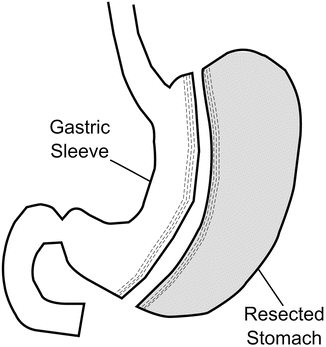

Fig. 1.3
Sleeve gastrectomy (SG)
An expert consensus statement was issued in 2011 to recommend best practice guidelines based on over 12,000 cases in an effort to reduce complications, improve efficacy, and move toward the adoption of standardized techniques and measures [64, 65]. Many recommendations were made. All panelists felt that use of a bougie was essential, while 87 % believed a 32 F–36 F to be the optimal size of the tubularized stomach. Most agreed that the closed height of the stapler should be at least 2.0 mm at the antrum and up to the incisura, while the closed height of the stapler beyond the incisura should be at least 1.5 mm. Additionally, it was felt to be important to fully mobilize the fundus before transection to prevent leaving behind too much stomach, particularly the fundus, which is relatively more distensible and may expand over time. Panelists also felt it was important to aggressively identify and repair any hiatal hernias. While the fundus should be fully mobilized, care should be taken to avoid stapling too near the gastroesophageal junction, as this may lead to narrowing of the esophagus or leaks at this point. Of note, many surgeons now feel that a bougie size of 40 Fr is preferred due to a potentially lower risk of leak [66].
EWL with sleeve gastrectomy ranges from 49 to 81 % [67]. The overall mean EWL 5 years or more after sleeve gastrectomy in a review of 16 studies was approximately 59 % [68]. Results from trials of LSG in patients with T2DM also show significant remission in the immediate postoperative phase. In observational cohorts, remission rates of T2DM are reported to range from 50 to 80 % at 12–18 months of follow-up [69–71]. The STAMPEDE trial compared outcomes between LSG and medical therapy, in addition to comparing RYGB to intensive medical therapy. At one-year follow-up, the rate of T2DM remission after LSG was 37 % versus 12 % for the medically treated subjects. Although the rate of remission of T2DM at 3 years was greater among patients undergoing RYGB (38 %) versus LSG (24 %), patients undergoing LSG still demonstrated a significantly higher rate of remission (p = 0.01) compared to patients who were treated with intensive medical therapy (5 %) [48, 50].
Based on the data of 12,799 laparoscopic sleeve gastrectomies from the International Sleeve Gastrectomy Expert Panel Consensus Statement of 2011, the average length of hospital stay after LSG was 2.5 ± 0.93 days. The conversion rate to open surgery was 1.05 % ± 1.85 %. The postoperative gastroesophageal reflux rate was 12.11 % ± 8.97 %. On average, patients experienced a 1.06 % leak rate and 0.35 % stricture rate [64]. The overall complication rate of LSG in large medical centers is < 15 % [72].
Despite a low overall mortality of 0.3 % with a leak-related mortality of only 0.1 % after LSG [66], the incidence of staple line leak and bleeding after LSG is perhaps the most concerning complication and potential target for technical improvement. Some controversy exists over the use of staple line reinforcement after sleeve gastrectomy [73]. A variety of surgical options including staple-line reinforcement, suture invagination, and biological sealant have been used to try and reduce the incidence of leak after sleeve gastrectomy. This topic will be discussed in greater depth in subsequent chapters.
1.7.3 Laparoscopic Adjustable Gastric Band Placement
This operation is often considered to be the least invasive bariatric procedure, although it does require the potentially permanent placement of a foreign body around the upper stomach. The first experiences in laparoscopic adjustable gastric banding (LAGB) were reported in 1993 [74–77]. Early approaches utilized the “perigastric” technique in which a retrogastric tunnel was created from the lesser curvature close to the gastric wall to the greater curvature about 2 cm below the cardia. Later approaches used the “pars flaccida” approach in which the pars flaccida was opened and a tunnel created behind the gastroesophageal junction above the level of the lesser sac. The adjustable silicone band could then be placed through this tunnel and secured anteriorly with a buckling device (Fig. 1.4).
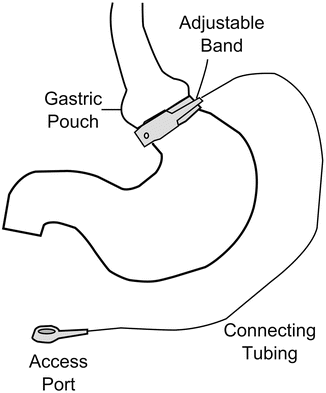

Fig. 1.4
Laparoscopic adjustable gastric band (LAGB)
For optimal results it is recommended that the pouch is sized to measure approximately 15 ml [78]. Additional recommendations include band imbrication anteriorly with two or more gastro-gastric sutures to prevent band slippage [78]. The band is then connected to an access port which is implanted in the subcutaneous tissue in the abdominal wall, allowing percutaneous inflation of the gastric band with saline for regulation of the opening of the ring. It is recommended to wait 4–6 weeks prior to band inflation in order to allow adequate healing of the stomach imbrications to reduce the risk of slippage [78].
The average EWL in patients undergoing LAGB is approximately 46 % [79, 80]. Variable rates of diabetes resolution after LAGB have been reported in the literature. In a recent meta-analysis, approximately 57 % of patients demonstrated resolution of diabetes after LGB [3].
In a randomized controlled trial comparing patients with mild obesity (BMI 30–35 kg/m2), significant differences in weight loss were observed at 2 years in patients who underwent LAGB (87.2 % EWL) when compared to a nonsurgical group who was treated medically (21.8 % EWL) [81]. They also observed a decrease in the rate of metabolic syndrome from 38 to 3 % in patients undergoing surgery compared to 38 to 24 % in medically treated patients [81].
In another randomized controlled trial comparing patients who underwent LAGB to those treated medically for type 2 diabetes and weight loss, significant differences were observed after 2 years with remission of diabetes in 73 % of patients undergoing LGB and 27 % of those undergoing conventional medical therapy [82]. Surgically treated patients lost a mean of 62.5 % of excess body weight compared to 4.3 % in the conventional-therapy group. Seventy percent of surgical patients experienced remission of metabolic syndrome compared to 13 % of the medically treated patients. Remission of type 2 diabetes was related to weight loss and lower baseline glycated hemoglobin levels. The authors concluded that weight loss after 2 years of treatment with LGB resulted in significant resolution of type 2 diabetes and metabolic syndrome in the majority of the obese patients with BMI < 40 kg/m2 when compared with medical treatment alone [82].
A randomized study from 2014 showed a more significant rate of diabetes remission among patients undergoing RYGB compared to those undergoing LAGB [83]. Rates of partial and complete remission of type 2 diabetes were 50 % and 17 %, respectively in the RYGB group and 27 % and 23 %, respectively, in the LAGB group (p < 0.001 and p = 0.047 between groups for partial and complete remission), with no remission in patients undergoing lifestyle and weight loss intervention [83].
Despite its low mortality and short-term morbidity, LAGB is associated with several late complications including band slippage, gastric erosion and gastric pouch dilatation [84]. Because of these issues, coupled with the reduced weight loss relative to gastric bypass and sleeve gastrectomy, and the “higher maintenance” required, the band has recently fallen out of favor and has been abandoned completely in many centers.
1.8 Biliopancreatic Diversion (BPD) and Biliopancreatic Diversion with Duodenal Switch (BPD-DS)
Scopinaro published his initial series of 18 patients undergoing biliopancreatic diversion in 1979 [85]. This procedure consists of a distal gastrectomy (antrectomy) leaving a proximal gastric pouch of about 200–400 ml volume [85]. The terminal ileum is divided 250 cm proximal to the ileocecal valve. The distal aspect of the divided ileum (alimentary limb) is brought up through a retrocolic tunnel and anastomosed to the remaining stomach. The proximal aspect of the divided ileum (biliopancreatic limb) is then anastomosed to the side of the distal ileum 50 cm proximal to the ileocecal valve, resulting in a common channel 50 cm in length. In a communication, Scopinaro reported an EWL of more than 70 % at 1 year and maintained for 20 years in the majority of the patients who underwent BPD [86].
In 1993, the BPD was modified by Marceau into the BPD-DS; he performed a vertical gastrectomy to create a gastric tube of approximately 200 ml volume based on the lesser curvature of the stomach rather than a horizontal gastrectomy as described by Scopinaro. With Marceau’s technique, the pylorus was preserved, the duodenum was cross-stapled and then the enteric limb anastomosed to the proximal duodenum [87]. However, a high rate of failures and weight regain were observed after disruption of the staple line of the duodenum and subsequent recanalization of the normal gastric-duodenal transit. In 1998, Hess described a comparable BPD-DS but with a division of the duodenum, closure of the duodenal stump, and end-to-end anastomosis of the enteric limb to the proximal duodenum [54].
The BPD-DS operation is technically demanding, particularly when performed laparoscopically, and is associated with a higher degree of protein, nutritional, and vitamin deficiencies than any other bariatric currently used procedure. However, it produces the greatest weight loss of any bariatric procedure and is the most likely to produce remission of diabetes. Studies suggest that mean EWL with BPD-DS at long-term follow-up ranges from 61 to 85 % [88–91]. In a systematic review, which included 48 studies for a total of 1565 patients comparing different bariatric surgical procedures, mean EWL at 2-year follow-up was 73 % with BPD-DS, 63 % with gastric bypass, 56 % with gastroplasty and 49 % with gastric banding. Diabetes resolution was greatest for patients undergoing BPD-DS (95.1 %), followed by RYGB (80.3 %), gastroplasty (79.7 %), and then LAGB (56.7 %). The proportion of patients with diabetes resolution or improvement was fairly constant at time points less than 2 years and 2 years or more [3].
In a prospective randomized controlled trial conducted among 60 patients by Mingrone et al., 95 % of subjects undergoing BPD achieved diabetes remission compared to 0 % in the medically treated group at 2-year follow-up. All patients had a history of at least 5 years of diabetes and glycated hemoglobin of 7.0 % or more. Remission was defined as a fasting glucose level of < 100 mg/dL and a glycated hemoglobin of < 6.5 % in the absence of pharmacologic therapy. There was also significantly greater improvement in total cholesterol levels, triglyceride levels and HDL levels among patients undergoing BDP versus medical therapy [49].
1.9 Emerging Bariatric Techniques
New bariatric devices and procedures intended to treat obesity are continually being developed. Endoscopic interventions such as transoral gastroplasty, the intragastric balloon, and the endoluminal gastrointestinal liner are a few devices that have gained recognition and demonstrated promising results [92]. Although these procedures are not as effective or sustainable at achieving weight loss as the surgical procedures that are being widely used, they have the potential to be less invasive, safer, and more cost-effective. These devices may hold potential for patients with early-stage obesity who do not yet qualify for traditional surgery; alternatively, they may serve as a bridge to traditional bariatric or non-bariatric operations for those who are too heavy to safely undergo surgery. Additionally such novel interventions may have potential as revisional procedures for failed bariatric surgical operations [93].
1.9.1 Intragastric Balloon
The intragastric balloon is one of the first endoscopic devices used for bariatric intervention. The balloon serves to reduce food consumption by occupying space in the stomach and inducing satiety. Since its inception in 1982, the intragastric balloon has undergone multiple transformations to minimize complications such as distal migration of the balloon leading to obstruction, ulceration and erosion, as well as nausea and vomiting that rarely require balloon removal. Earlier devices were designed as single balloons composed of silicone, which were inflated with approximately 400–700 ml of saline after being endoscopically deployed in the stomach. This type of balloon was removed from use in the USA due to problems with the complications noted above.
A newer model introduced by ReShape Medical (San Clemente, CA) and branded as the ReShape Duo is a dual-balloon device that is filled with 900 ml of saline and is designed to maximize space occupation in the stomach. This newer device potentially reduces the undesirable risk of migration, obstruction, and perforation conferred by the single balloon. If one balloon deflates in a dual-balloon device, the second balloon will maintain the device within the stomach, preventing migration and possible bowel obstruction while allowing the patient enough time to seek medical attention. The intragastric balloon is typically left in place for 6 months after which it is endoscopically deflated and extracted using a snare or basket [94].
Several investigators have evaluated the safety and efficacy of the intragastric balloon in the management of obesity. In the largest reported study retrospectively analyzing the results of the intragastric balloon, the 6-month EWL was 33.9 % ± 18.7 % in 2515 patients. Patients with hypertension and diabetes achieved significant improvements in blood pressure and glycemic control. The authors reported five cases of gastric perforation (0.19 %), 2 of which were fatal [95]. Similar results were seen in a meta-analysis of 15 studies which demonstrated a 32 % EWL with a 0.1 % incidence of gastric perforation [96].
The intragastric balloon has also been used as a bridge for super-obese subjects with multiple medical comorbidities, allowing the achievement of short-term weight loss and reduction in comorbidities, potentially reducing the risk for subsequent traditional bariatric surgery [97]. The greatest pitfall of the device is the durability of weight loss. In a study looking at patients at approximately 5-year follow-up, only one-fourth of subjects sustained weight loss in the absence of any dietary or exercise regimen after balloon removal [98]. The Reshape balloon was approved by the FDA in July, 2015 for use in adult patients with a BMI of 30–40 kg/m2. It remains to be seen whether the device will achieve significant clinical acceptance.
1.9.2 Duodenojejunal Bypass Sleeve
The endoscopic duodenal-jejunal bypass liner (EDJL), also known as the Endobarrier Gastrointestinal Liner (GI Dynamics Inc., Lexington, Mass), is a 60-cm impermeable fluoro-polymer liner, which is placed endoscopically and anchored at the duodenal bulb. Ingested food and gastric secretions pass through the interior of the liner while pancreatic enzymes and bile acids are diverted around the exterior of the liner. The EDJL is a temporary device, designed to be left in place for 6 months before endoscopic removal. The sleeve is intended to mimic the effects of gastric bypass surgery by delaying digestion and intervening with the body’s metabolic functions, including alteration of incretin pathways [93].
In a multicenter, randomized clinical trial conducted in the Netherlands, 30 patients underwent EDJL and 11 were designated to adhere to a low-calorie diet alone. The mean percentage of EWL after 3 months was 19.0 % for patients who underwent a EDJL compared with 6.9 % for control patients (P = .002). All patients in the EDJL group had at least one adverse event, such as nausea, upper abdominal pain, pseudopolyp formation, or implant site inflammation. There were no serious adverse events, and all minor adverse events resolved either spontaneously of after temporary medication with no further sequelae [99]. Other studies have also demonstrated the efficacy of the EDJL in achieving significant weight loss; nonetheless, all series reported some degree of adverse events, a proportion of these classified as major adverse events, such as upper gastrointestinal bleeding, anchor migration, and stent obstruction [100].
A recent study explored the potential for EDJL in managing type 2 diabetes mellitus. At 1 year, patients who underwent the endoscopic procedure were found to have significantly lower requirements for insulin therapy than those who were treated with dietary interventions. Baseline glycated hemoglobin levels were 8.3 % for both groups and dropped to 7.0 % and 7.9 % in patients who underwent EDJL and dietary intervention, respectively (p < 0.05) [101]. The EDJL is approved for use in Europe to treat patients with type 2 diabetes mellitus and obesity for 12 months. However, the US pivotal trial of the device was terminated in July, 2015, due to higher than expected rates of hepatic abscess in clinical subjects. With the premature conclusion of this trial it appears unlikely that the device will ever see clinical use within the USA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







