Fig. 22.1
(a, b) The microconvex probe handled by the surgeon during resection area demarcation and the corresponding ultrasound imaging with a large scanning window, (c, d) a new T-shaped probe with trapezoid scanning window designed by the surgeon to be easily handled and to allow surgical maneuvers, electrocautery (EC), tumor (T), finger (F), portal branch to the segment 7 (P7), portal branch to the right posterior section (P6-7), portal branch to the right anterior section (P5-8)
22.3 Technical Tricks of Liver Exploration
The ultrasound machine should be placed in front of the first operator with the aim to simultaneously check the screen and the operation field. The screen must be large enough for an optimal visibility at that distance, and the assistants should then control the machine’s keyboard that can be covered with a sterile drape. The liver exploration should always start with the inspection and palpation of the entire peritoneal cavity: this should not be avoided even with IOUS because inspection and palpation still play a relevant role [4]. Liver mobilization starts with the division of the round and falciform ligaments as well as of adhesions to free the liver surfaces in order to get enough space for handling of the probes for exploration by IOUS. Thus, pulling the round ligament, the liver surface is widely exposed, and following the examination of the portal branches and hepatic veins, the whole liver can be studied.
22.4 Diagnosis and Staging of Benign Lesion
The two main targets of IOUS explorations are represented by detection and differentiation. Particular attention has to be paid in those patients with multinodular malignant disease in which some benign lesions could be misdiagnosed as part of the neoplastic involvement or in those patients who are going to be resected for liver neoplasms which at preoperative imaging resulted as carrier of benign lesions which have to be confirmed intraoperatively as really benign.
Proper interpretation of the echo pattern of a lesion is of paramount importance. Simple hepatic cysts are frequently encountered, and they appear typically as well-defined anechoic (black) lesion, with very tiny or unapparent wall, and a posterior acoustic shadow which exists also when they are very small (Fig. 22.2). Inversely, this phenomenon is absent if the lesion is a solid one as in the case of tiny liver metastasis (Fig. 22.3). However, it should be noted that the misinterpretation may result if the IOUS image is not properly set and in particular the gain and the focus are not correctly adjusted. Therefore, the gain of the gray scale should not be too high, and the focus level should not be positioned distant from the level of the target; otherwise, a cystic lesion may appear as a solid one (Fig. 22.4).
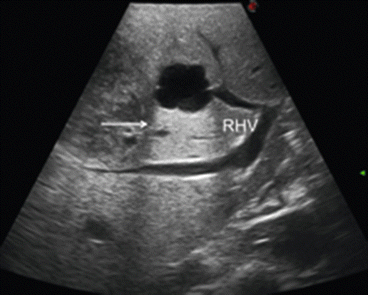
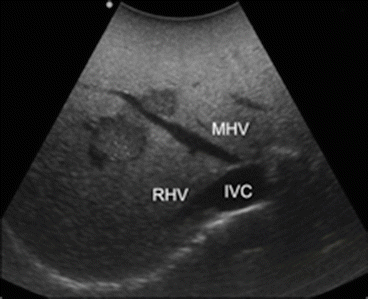
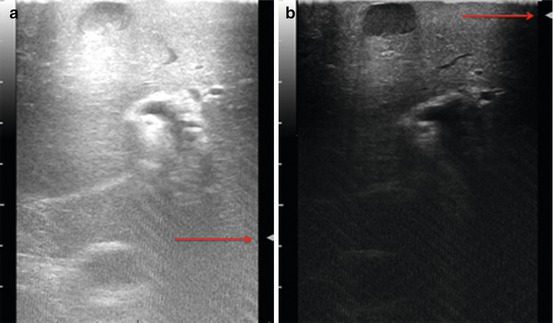

Fig. 22.2
Ultrasound appearance of a simple hepatic cyst with anechoic content and its posterior wall echo (arrow), right hepatic vein (RHV)

Fig. 22.3
Two small colorectal liver metastases with hypoechogenic pattern, right hepatic vein (RHV), middle hepatic vein (MHV), inferior vena cava (IVC)

Fig. 22.4
(a) Example of ultrasound image not adequately set with focus (arrow) level incorrectly positioned (deep) in accordance to the lesion location (superficial) and with high gain regulation (high brightness level) where a typical hepatic cyst mimics a solid lesion, (b) the typical ultrasound appearance of the hepatic cyst is here revealed once the image equalization is adequately set and the focus correctly located based on the location of the target lesion
Furthermore, occasionally the round ligament may be unexpectedly prominent, raising the suspicion of a solid hepatic mass appearing in a transverse scan as a hyperechoic target lesion: shifting to a sagittal scan helps in disclosing its linear shape (Fig. 22.5).
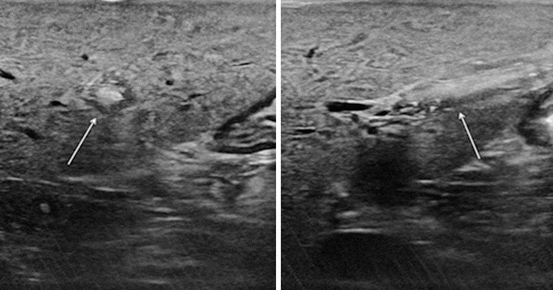

Fig. 22.5
(left) Particularly prominent round ligament (arrow) simulating an intrahepatic echogenic mass, (right) changing the transducer axis of the origin of the ligament from the umbilical portion and its linear shape is revealed
A frequent source of misinterpretation is also represented by the focal steatosis areas, consisting in portions of the liver with localized accumulation of hepatocytes with abnormal distribution of cytoplasmatic lipid shell [5], resulting in areas with different echogenicity. In some cases this focal accumulation can assume a nodular shape which could be misleading. However, there are some features which should secure from misinterpretation: indeed, these areas are typically located around the gallbladder or around the main portal pedicles, have an irregular shape, and present vessels passing through without distortions (Fig. 22.6).
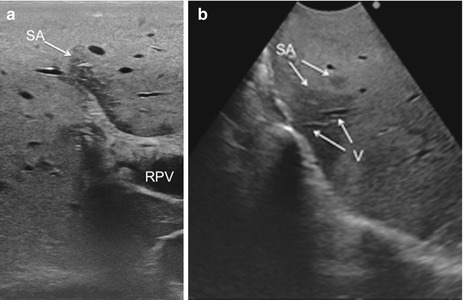

Fig. 22.6
Focal fatty infiltration area, also called “skip area,” typically located around the main portal pedicles (a) and featured as irregularly shaped with vessels running through without structural distortion or interruption (b); skip area (SA), vessel (V), right portal vein (RPV)
Finally, there is an artifact being a true optical illusion which is called the mirror effect and can mimic an additional lesion generally outside the organ. This typically happens when a lesion is just superficial and adjacent to the diaphragm (Fig. 22.7).
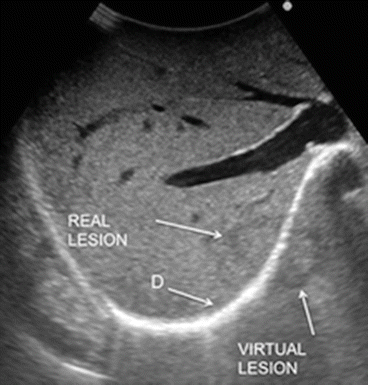

Fig. 22.7
Typical appearance of the so-called mirror effect obtained for a lesion just adjacent to the diaphragm in which a duplicated lesion is visualized; diaphragm (D)
IOUS allows the detection of new lesions in around 30 % of cirrhotic patients: most of the nodules, however, detected in these patients are nonneoplastic with thereby the risk of overestimating the tumor stage. Indeed, excluding those nodules with mosaic ultrasonographic patterns, typical of hepatocellular carcinoma (HCC), which are malignant in 84 % of cases, only 24–30 % of hypoechoic nodules and 0–18 % of those hyperechoic are malignant [6, 7]. To overcome this problem, even biopsy may not be adequate. The only nodule which can be easily differentiated intraoperatively from an HCC or a liver metastasis is the tiny hemangioma which is not infrequently encountered primarily during IOUS exploration: it has a typical ultrasonographic pattern including a uniform hyperechogenicity, well-defined margin, and posterior echo enhancement and moreover when compressed changes its size and shape (Fig. 22.8). This feature is based essentially on the different stiffness of the hemangioma compared to other lesions. Stiffness measurements are advantageous in this respect. Recently, intraoperative elastography has been applied to the clinical practice as promising imaging method for differential diagnosis of hepatic tumor. In fact, elastography can provide information about the lesion differentiation based on tissue elasticity expressed on the IOUS screen by differences in colors. As reported, some authors have introduced a classification for differentiating HCC from metastatic lesions based on their tissue elasticity at intraoperative elastography (Fig. 22.9) [8].
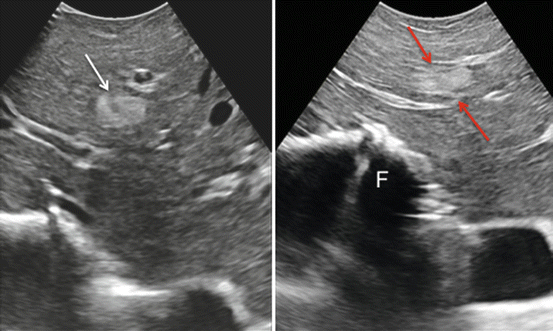
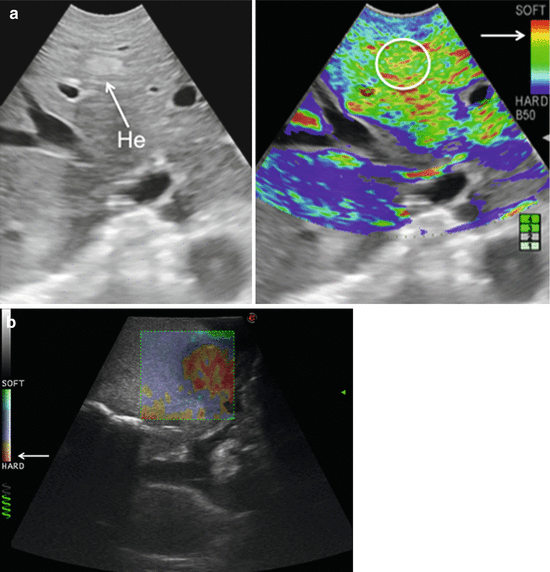

Fig. 22.8
(left) A hemangioma with typical uniform hyperechogenicity (arrow); (right) the diagnosis of hemangioma is here confirmed since once compressed under IOUS guidance, the lesion changes its shape and thickness (arrows); finger (F)

Fig. 22.9
(a) Comparison of elastogram (right) and B-mode ultrasound scan (left) in a patient with small hemangioma (circle): the real-time intraoperative elastography confirmed the lesion softness according to the color scale (arrow); (b) hardness of a colorectal liver metastasis is here confirmed by the real-time elastography based on its predominantly red color according to the color scale (arrow), hemangioma (He)
Focal lesions which liver surgeons now more frequently should need to address in patients affected by colorectal liver metastases are the focal nodular hyperplasia (FNH). FNH is a benign condition that occurs in normal liver with prevalence up to 3 %, as reported in autoptic studies [9, 10]. Recently, the extensive use of oxaliplatin-based systemic chemotherapy to treat patients who are carriers of colorectal cancer has been observed to be associated with the development of liver FNHs with a prevalence up to 15 % of the cases [11]. As reported, small FNHs arising during systemic treatment may have preoperative imaging appearance which overlaps with those of metastatic lesions [12]: in this way, the preoperative diagnostic modalities introduce the risk of overestimating the disease or postoperatively the incidence of recurrences in patients resected of CLM. Thus, IOUS exploration of the liver remains crucial. In this sense, if a strict parenchyma-sparing approach is attempted, the attention paid to discriminate eventual new lesions is probably more pronounced than in case of preference of a conventional surgical approach such as major or extended hepatectomy.
Indeed, larger FNH are less problematic for diagnosis because the capability of diagnostic imaging is higher, with proven sensitivity greater than 90 % [13, 14], in detecting the typical central scar which is distinctive of this liver entity. Sometimes, however, the central scar is so small in size to be easily detected by ultrasound (Fig. 22.10): the use of color Doppler, providing the presence of the central feeding artery, improves the detection and the characterization of this kind of lesion (Fig. 22.11).
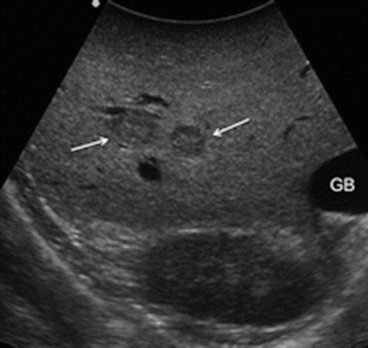

Fig. 22.10




Example of patient treated with oxaliplatin-based systemic chemotherapy with very small focal nodular hyperplasias (arrows) with an hypoechoic pattern and a central scar not detectable by IOUS which mimics a small liver metastasis; gallbladder (GB)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






