Fig. 21.1
Celiac angiography shows the typical appearance of FNH as a hypervascular lesion with a central feeding artery radiating in a spoke-wheel manner into the lesion (Reprinted with permission from Terkivatan et al. [50])
Cavernous hemangioma usually shows contrast-containing vascular spaces orderly in their configuration which persist into the venous phase [51] (Fig. 21.2). HCA appears usually hypervascular [53] (Fig. 21.3), while PLD shows arteries and portal veins coursing not parallel, as usual, but in a different fashion: almost all hepatic arterial branches are well developed, whereas portal venous branches in the cystic segments are obstructed and replaced by multiple cysts [49] (Fig. 21.4). Arteriovenous shunting with early opacification of the hepatic veins or of the portal system must be considered to avoid an accidental embolization of nontarget vessels [14].
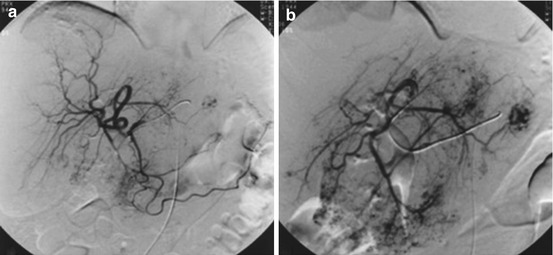
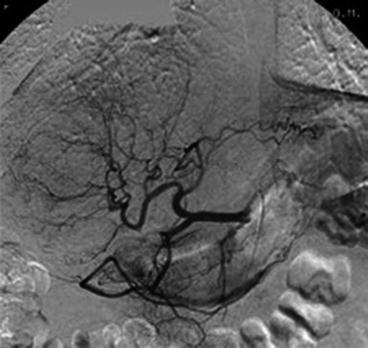
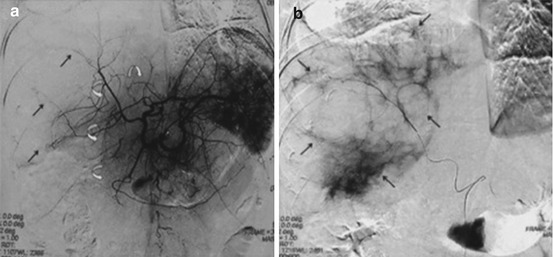

Fig. 21.2
DSA of a giant cavernous hemangioma in the right liver lobe. (a) Right hepatic arteriogram. (b) Superselective catheterization of the feeding artery (Reprinted with permission from Giavroglou et al. [52])

Fig. 21.3
Selective angiogram of the common hepatic artery shows a large adenoma in the right liver lobe. Pathological branches and splaying of normal branches by the adenoma are observed (Reprinted with permission from Erdogan et al. [53])

Fig. 21.4
Symptomatic polycystic liver disease: (a) Celiac arteriography shows that the right hepatic arterial branches are well developed and stretched (arrows), representing cystic regions. The left hepatic arterial branches are relatively normal (curved arrows), representing nearly intact hepatic parenchyma. (b) Superselective right hepatic angiography shows the multiple right lobe liver cysts (arrows) (Reprinted with permission from Wang et al. [47])
In venous phase, a preserved opacification of the portal vein represents an essential condition to perform a TAE: an unknown portal vein thrombosis can potentially contraindicate a TAE due to the potential risk of a hepatic insufficiency.
While the goal of an elective TAE in benign liver tumors is to obtain a significant shrinkage of the lesion, minimizing its related symptoms, in emergency the aim of TAE is to stop an active arterial bleeding. The risk of concomitant ischemic damage of liver parenchyma remains low because of the portal vein tributaries accounting for 70 % of the total blood supply of the liver. However caution is necessary to preserve the major liver arteries avoiding unnecessary liver damage. Selective or superselective catheterization of the hepatic artery and its distal branches using dedicated catheters and microcatheters and adequate embolizing material is essential. The current use of flexible microcatheters of small caliber (less than 3 French) but with adequate diameter of their internal lumen (0.025–0.028 in.) determines several advantages: it allows great trackability, distal superselective catheterization, good diagnostic capacity (adequate quantity/flow of contrast agents can be injected), and low rate of microcatheter subocclusion due to embolizing material passage. Various embolizing materials can be used to occlude the feeding vessels of a lesion. The materials for embolization should be easy to use and capable of occluding the supplying vessel without compromising the flow of the main liver arteries [53]. Different materials, to perform proximal or distal TAE, are available: devices, as coils or Amplatzer plugs, which are pushed and released adjacently to the (micro)catheter tip and particles or solutions, as polyvinyl alcohol (PVA) particles, microsphere, gelatin sponge, glue, N-butyl cyanoacrylate (NBCA), liquid polymeric embolic system, and ethanol, which are injected and carried with the blood flow in the lesion microcirculation. Metallic coils of different diameters (0.035, 0.025, and 0.018 in.) and lengths, if used alone, often prove insufficient in blocking the peripheral arterial flow due to recanalization and collateral formation (Fig. 21.5); sometimes the use of Amplatzer plug [14] can represent a good solution in case of arteriovenous shunting or to avoid a nontarget vessel embolization. However metallic coils have been previously used in TAE of HCA [53] and PLD [54]. Gelatin sponge has been commonly used for embolization of arterial bleeding [53], but its temporary nature leaves a high risk of collateralization. PVA particles of different sizes (150–250; 250–350; 300–500 μm) have been largely used in TAE of giant hepatic hemangiomas [51, 52, 55], FNH [39, 50], and HCA [53]. The wide use of PVA particles in TAE of benign liver tumors is due to their long-term occlusive properties, efficacy in occluding small arteries, and biocompatibility [52] (Fig. 21.6). NBCA mixed with iodized oil [47] has been used to reduce hepatomegaly in PLD: unlike coils, which are solid, NBCA is deployed in a liquid form before polymerization, and this feature is beneficial in occlusion of smaller vessels.
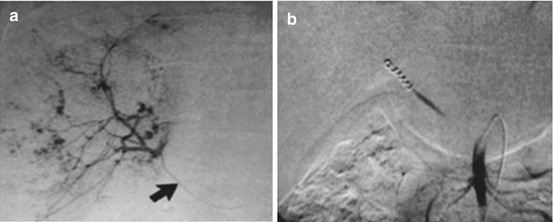
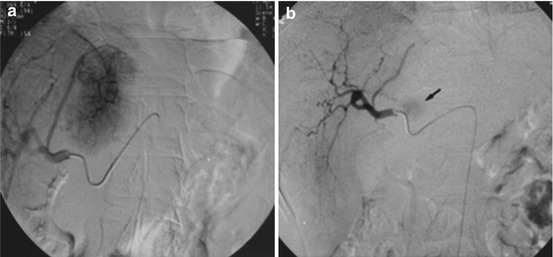

Fig. 21.5
Large cavernous hemangioma in the right lobe of the liver. (a) The angiographic catheter (arrow) in the hepatic artery arising from the superior mesenteric artery shows a large cavernous hemangioma supplied by the right hepatic artery. (b) The tumor was embolized with multiple steel coils and, successively, polyvinyl alcohol particles (Reprinted with permission from Srivastava et al. [28])

Fig. 21.6
(a) DSA shows a hypervascular lesion (FNH) with a microcatheter placed in the left hepatic artery, which supplied the tumor. (b) Angiographic image after the embolization procedure of the lesion by using embolization particles (contour; diameter 150–250 μm). After the procedure a small part of the lesion remains (arrow) (Reprinted with permission from Terkivatan et al. [50])
The association of two embolizing materials, for example, coils and PVA particles, can be useful in selected cases to simultaneously perform a proximal and a distal embolization.
Finally, the choice of the embolizing material depends on many factors: lesion characteristics, angiographic evidence, interventional radiologist experience, and elective or emergent procedure (Fig. 21.7).
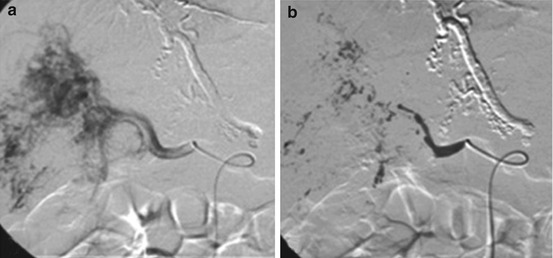

Fig. 21.7
Catheter embolization in infantile hepatic hemangioma. (a) DSA with coaxial technique with microcatheters shows a large hemangioma in the right lobe. (b) Especially when intrahepatic macroshunts are present, proximal embolization of feeding vessels with glue is effective (Reprinted with permission from Temple and Marshalleck [49])
21.1.3 Complications
Complications of vascular embolization include the use of intra-arterial contrast medium, procedure-related complications, and postembolization syndrome. The postembolization syndrome usually occurs within 24–48 h of embolization and lasts 3–7 days. It is characterized by pain at the site of embolization, nausea and vomiting, malaise, fever, and leukocytosis. Severity depends on the size of the embolized tissue [39]. The main procedure-related complications are represented by liver abscess, developing in the necrotized tissue [56], biliary damage [57], and systemic infections [58]. Therefore, a strict aseptic approach is essential, and, if necessary, prophylactic antibiotics can be discussed. Prophylactic antibiotics should be administered in case of previous bile duct surgery or systemic disorders. Further complications are accidental tissue necrosis of adjacent organs due to either reflux of embolizing material or permeation of liquid embolic material into the capillary bed. Renal failure may occur if a large mass of tissue is infarcted due to toxic radical release [39]. Other possible complications are pneumonia, pleural effusion, ascites, increased serum transaminases, and hepatic insufficiency.
21.2 Ablation
Basing on the mechanism to cause injury, local ablation techniques can be divided as follows: thermal therapies such as radiofrequency ablation, percutaneous cryoablation, microwave ablation, and interstitial laser chemotherapy and chemical ablative techniques such as percutaneous ethanol injection and electroporation, which alters the electrical conductivity and permeability of the cell membrane [59].
Radiofrequency ablation (RFA) is one of the main local treatment strategies that can be used for the destruction of a variety of unresectable malignant (and benign) liver tumors. Hepatic RFA is less invasive than surgery and carries a low risk of major complications [60]. RFA has gained universal acceptance as a safe and effective method to treat unresectable liver tumors showing excellent local tumor control and survival in different liver tumors [61, 62]. The use of RFA determines hyperthermia-induced coagulation necrosis of the hepatic lesion which typically exits as an area (RFA zone) demonstrating a defect in contrast enhancement at CT, MR, and CEUS [63]: according to the American Heritage Medical Dictionary, the term coagulation necrosis is defined as “necrosis in which the affected cells or tissue are converted into a dry, dull, fairly homogeneous eosinophilic mass as a result of coagulation of proteins” [64]. This definition is a description of the microscopic tissue change; in the case of RFA-induced coagulation necrosis, the protein coagulation is induced by hyperthermia. With the use of current RFA technologies, the area of coagulation necrosis, the RFA zone, is oval or round and can measure approximately 5 cm in diameter. However, the size and the shape can vary according to many factors, including the electrode type, the duration of ablation, and hepatic perfusion [65]. The clinical role of RFA of the liver has been well established, and its overall therapeutic efficacy, especially in cases of hepatocellular carcinoma (HCC), is known to be comparable to that of surgical resection as long as the tumor size is sufficiently small [66]. The role of RFA in the management of benign liver tumors has been more recently described in adults and children in selected cases [67]. RFA can be performed using a laparoscopic approach [68] or a percutaneous approach, with ultrasonographic guidance [69] or, more rarely, with CT guidance [70]. In the treatment of large benign liver tumors, as, for example, symptomatic hepatic hemangiomas with diameter over 10 cm [68–70], large and separate ablation sessions with clustered electrodes and multiple overlapping treatment zones can be useful to reduce the prevalence of postablation syndrome and risk of treatment failure.
21.2.1 Indications
RFA represents an emerging interventional procedure increasingly used in the treatment of malignant and benign liver tumors as valid alternative to surgery.
Indications to RFA for treatment of benign liver tumors are represented by symptomatic nodules or at high risk of malignant degeneration or rupture.
The main benign liver tumors treated with RFA are hepatic hemangiomas [69–71], HCA [72, 73], and, more rarely, also FNH [74].
Cavernous Hepatic Hemangioma
Differently from small and asymptomatic hepatic hemangiomas, hepatic cavernous hemangiomas of diameter superior to 5 cm can be symptomatic with abdominal discomfort, swelling, abdominal pain, jaundice, and thrombocytopenia [68]. Giant hepatic hemangiomas can also have high risk of rupture [75]. Besides surgical resection [23], management of symptomatic, increasing in volume, large hepatic hemangiomas has been based on RFA, TAE [27–29], steroid treatment [21], radiation therapy [22], and hepatic arterial ligation [24]. The RFA mechanism of action in the treatment of hepatic hemangiomas is still not completely clear. Most of hepatic hemangiomas are of the cavernous type, composed of widely dilated nonanastomotic vascular spaces lined by flat endothelial cells and supported by fibrous tissue. RFA is a localized thermal technique designed to destroy tumors by using radiofrequency energy to heat tumor tissue to temperatures exceeding 90 °C. Damage to the endothelium lining the vascular structures may promote thrombosis [68]. The site of the hepatic hemangioma determines different approaches: laparoscopic approach in case of subcapsular lesion or percutaneous approach for deeper tumors. Ultrasonography represents the main standard method of guidance of RFA, but also CT guidance, in case of ultrasonographic poor visibility, has been described [70]. Most of hepatic hemangiomas show hyperechoic pattern at ultrasonography.
In case of giant hepatic hemangiomas, the RF electrode is repositioned to fully cover the hemangioma without ablating the surrounding liver tissue according to the overlapping ablation method. The total number of puncture of RFA can exceed 5 until 8 [68]. The benign biologic nature and high vascular properties of hepatic hemangiomas determine more advantages than disadvantages. The advantages include the following three aspects. First, differently from malignant tumors, RFA for hepatic hemangiomas does not require an ablative margin of the normal hepatic parenchyma surrounding the tumor. Second, one well-performed RFA can lead to an obvious collapse of tumor tissue around the ablation zone, making RFA for hepatic hemangiomas be more efficient and easier than for malignant tumors. Third, the residual hepatic hemangioma tissue after RFA does not necessarily need a prompt further treatment due to the slow tumor progression and the inability to metastasize.
The main disadvantage, especially for giant hepatic hemangiomas with diameter more than 10 cm, is their generous blood supply which is responsible for hemolysis and subsequent hemoglobinuria, hemolytic jaundice, anemia, and, in severe cases, renal damage [70].
Hepatocellular Adenoma (HCA)
HCA is a benign liver tumor histologically characterized by closely approximated cords of hepatocytes which have vacuolated sinusoidal borders. They are usually solitary nodules variable in size. HCA occurs primarily in young women; it is associated with oral contraceptive use, diabetes, and type 1 glycogen and can develop during pregnancy. Treatment is recommended in selected patients due to the risk of life-threatening hemorrhage and malignant transformation [40]. TAE and RFA are less invasive therapeutic options than surgical resection, which remains the gold standard treatment. Usually percutaneous RFA for HCA is performed in children [72, 73] and adults [76, 77] under ultrasonographic guidance. Most HCAs show hypoechoic pattern at ultrasound. RFA can be performed safely with a good local tumor control and low morbidity. In case of liver adenomatosis, RFA can have an important role as concurrent treatment with surgical resection [78]. As limit, RFA is not able to make large-volume ablations in a single session.
21.2.2 Technical Aspects
Image-guided percutaneous RFA is a minimally invasive, relative low-risk procedure for the treatment of malignant and benign liver tumors. Local recurrence and survival rate depend on complete ablation of the entire tumor including a sufficient margin of surrounding healthy tissue. Currently a variety of different RFA devices are available. Tumors may currently be approached either via use of an expandable needle electrode that deploys multiple hooks into a lesion or via a straight-tip internally cooled electrode. In RFA, the high-frequency alternating current from the electrode generates marked agitation of the ions in the tissue that surrounds the uninsulated tip of the probe. The frictional heat results in thermal coagulation necrosis of the surrounding tissue. The size of ablation correlates with the intensity and duration of energy deposition. In order to achieve an effective heating throughout the tumor, a temperature of 60–100 °C has to be maintained throughout the entire target volume at least for 4–6 min, but the duration of application may increase to 10–30 min. The flow of current and heat absorption depend on tissue characteristics and perfusion [79]. Thus, the coagulation size may be increased by reduction of the hepatic perfusion during RFA by temporary occlusion of the portal vein or the hepatic artery [80, 81]. Another key component for successful tissue destruction involves the technique of overlapping ablations, as, for example, in RFA treatment of giant hepatic cavernous hemangioma. Treatment of even larger lesions may be attempted via creation of spherical zones of overlapped ablation to create a cylinder (“thermal cylinders”). These cylinders are then overlapped to systematically ablate a larger tumor [82]. Differently from HCC, in the RFA of HCA, the ablation zone can be limited to the nodule without extending to the surrounding healthy hepatic tissue. Accurate planning and execution of RFA according to the size and geometry of the tumor is essential. In order to minimize complications, individualized treatment strategies may be necessary for tumors close to vital structures [83].
Liver tumor RFA can be carried out by a percutaneous, laparoscopic, or laparotomic approach. The guidance system is generally represented by ultrasound [84] (Fig. 21.8). In case of unsatisfactory sonographic window, recent alternatives to ultrasound guidance are CEUS [85] and CT fusion-imaging guidance [86, 87]. CEUS, CT, and MR examinations are the most sensitive test for assessing therapeutic results [88].
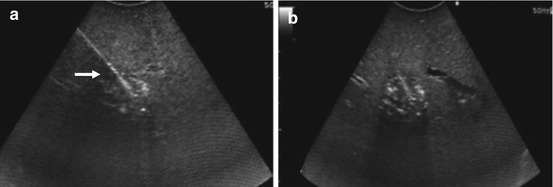

Fig. 21.8
US-guided radiofrequency ablation of a small HCA. (a) The needle tip (arrow) is in the center of the lesion to be ablated. (b) Ablation in progress: tumor and surrounding tissue are going through tissue necrosis and coagulation. As the tissue is undergoing severe heat conditions, the bleeding vessels are seared, and microbubble formation appears as a hyperechoic cloud around the needle tip
Percutaneous RFA can be performed with local anesthesia and mild sedation; deep sedation or general anesthesia is also used. The skin is pricked with a small lancet, and the active electrode needle tip is introduced into the tumor under imaging guidance. The access is intercostal or subcostal. If an expandable electrode is used, the hooks are deployed after needle placement and retracted before withdrawing it. If a cooled electrode is employed, the pump is switched on, and chilled sterile saline circulates through the needle. At the end of the procedure, the RFA generator is reactivated to obtain a hot withdrawal so as to prevent oozing and tumor seeding. For RFA of very large tumor as giant hepatic cavernous hemangiomas with diameter more than 10 cm, the application of bipolar RFA can be useful to obtain a significant shrinkage of the mass: an important disadvantage of the bipolar RFA system is the relative unsafeness of the percutaneous approach (asymmetrical axis of 8 × 14 gauge instead of the traditional 15–18 gauge) which makes an open approach preferable [89].
In addition to size, tumor location is one of the most important factors influencing efficacy and complication rate of RFA. For example, if the tumor lies in the subcapsular area, no safety margin along the capsule is possible. In addition tumor in the central zone of the liver near the hepatic hilum is almost always surrounded by large vessels that, because of the heat-sink effect of flowing blood, make it difficult to ablate the periphery of the tumor and the cuff of normal hepatic tissue [82]. In these situations, which can potentially contraindicate RFA, different strategies are needed to obtain successful treatment and minimize complications, for example, the creation of an artificial ascites with saline solution local injection before RFA of a subcapsular lesion [90].
21.2.3 Complications
Complications of RFA can be classified in two general categories: those related to imaging-guided electrode placement and those related to thermal treatment itself [60]. Complications in both categories can be major or minor. A major complication, as defined by Society of Interventional Radiology, is any post RFA complication that (a) requires therapy with minor hospitalization (<48 h); (b) requires major therapy, an unplanned increase in the level of care, or prolonged hospitalization (>48 h); (c) results in permanent adverse sequelae; or (d) leads to death [91]. All other complications are considered minor and include anything that either (a) does not require therapy or (b) requires nominal therapy, with overnight admission for observation only. In a large study of 3,554 treated hepatic tumors, Livraghi et al. [92] reported major complications in 2.2 % of cases and minor complications in less than 5 % of cases, with a death rate of 0.3 %.
Complications related to imaging-guided electrode placement are the following: intraperitoneal or intrahepatic bleeding; vascular complications, including arteriovenous fistula, pseudoaneurysm, thrombosis, stenosis, and infarction; pneumothorax; hemobilia; and grounding pad burns.
Complications related to thermal treatment include injury to nontarget organs as bowel, gallbladder, bile ducts, and diaphragm.
Other eventual complications are represented by infection, postablation syndrome, fulminant liver failure, tumor seeding, and treatment failure.
Bleeding, often self-limiting, represents one of the most common complications and occasionally can require endovascular or surgical management, in case of hemodynamic instability of the patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






