Infectious
Noninfectious
Parasitic
Nonparasitic
Simple cyst
Partial cystic component
Hydatic cyst
Pyogenic liver abscesses
PCLD
Intrahepatic pseudocysts
Amebic abscess
Peribiliary cyst
Bilomas
Post-traumatic hematoma
Surgical treatment should only be reserved for really symptomatic patients because approximately 25 % of patients who undergo surgery for referred symptomatic benign liver lesions remain somehow symptomatic even after surgery [4].
8.2 Simple Cyst
Simple hepatic cyst is a biliary malformation, which, generally, does not have communication with the intrahepatic biliary tree. Most cysts measure less than 3 cm and are asymptomatic [2].
Microscopically, they are lined by a single layer of cuboid or columnar epithelial cells, resembling biliary epithelial cells. Its origin is derived from aberrant bile ducts that have lost communication with the biliary tree and continue to secrete intraluminal fluid [4].
The female-to-male ratio is 1.5:1, and prevalence is from 2.5 to 18 % at ultrasound studies with a more frequent incidence in adults over 50 years, although the number of clinically relevant cysts , however, remains low [4, 5]. The rate of single cysts is about 68–75 % [5, 6].
8.2.1 Pathogenesis and Epidemiology
Simple cysts contain a clear, bile-like fluid. Because bile duct epithelium covers the simple cyst inner lining, it is hypothesized that simple cysts arise during embryogenesis when intrahepatic ductules fail to connect with extrahepatic ducts [7–10]: these aberrant ductules eventually dilate to form simple cysts.
8.2.2 Symptoms
Less than 4 % of simple cysts are almost always asymptomatic, but larger cysts greater than 5 cm especially when they reach a size over 10–12 cm and, particularly, are located in the left or anterior segments may cause compression of the stomach (Figs. 8.1 and 8.2) or modification of the abdominal profile with evident symptomatic manifestations [11, 12].
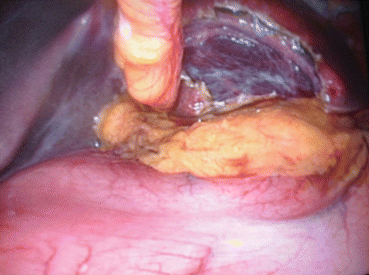

Fig. 8.1
Large simple cyst of the segment III with stomach compression

Fig. 8.2
After fenestration evidence of compression of the stomach
A not so rare complication is intracystic bleeding, generally due to an overwhelming size (over 15–20 cm). A surgical treatment is evidently indicated in this case.
Case Report 1
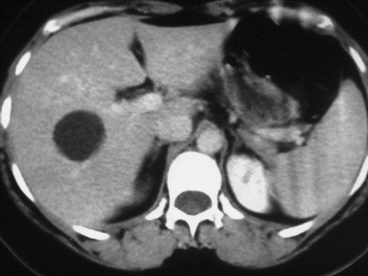
A simple cyst of segments 5–8 in a 35-year-old female patient with an acute pain on the upper right abdomen and loss of hemoglobin. CT scan (Figs. 8.3 and 8.4) showed a rapid volume increase in 25 days due to an intralesional hemorrhage. The patient was treated by laparoscopic fenestration with no recurrence after 4 years.
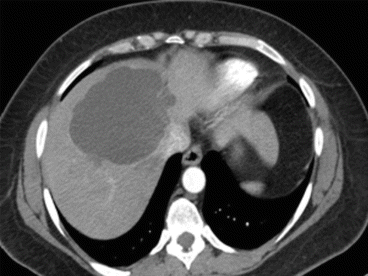
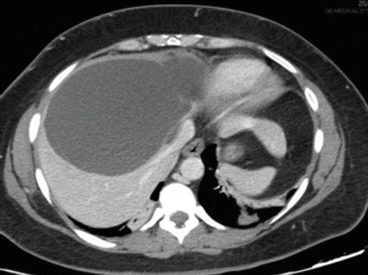

Fig. 8.3
First CT scan of case report 1

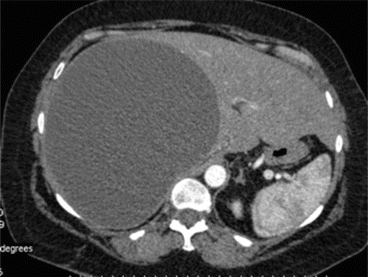
Fig. 8.4
CT scan of case report 1 at 25 days after first CT scan
8.2.3 Instrumental and Laboratory Diagnosis
Almost all patients have liver function tests within the normal range.
Ultrasound (US) is the best imaging modality to study simple cysts , which appear as a circular or oval, anechoic lesion with smooth borders and acoustic posterior enhancement and, in most cases, without internal septations [13] (Fig. 8.5). Also, ultrasound is strongly advised to monitor the evolution of these cysts: generally one time a year. Cysts that are found less than 3 cm at first diagnosis generally do not grow even in young patients. But we have been able to ascertain occasionally unexpected growth in size of small cysts. And therefore instrumental follow-up is generally advisable.
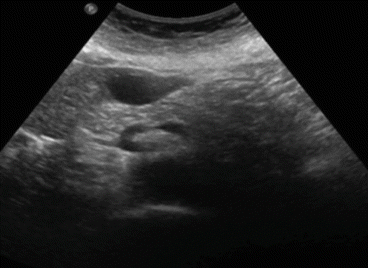

Fig. 8.5
Ultrasound image of a simple liver cyst of segment III (4 cm in diameter)
Case Report 2
Further imaging studies, including computed tomography (CT scan) (Fig. 8.8) or magnetic resonance imaging (MRI) (Fig. 8.9), are not routinely required and show a nonseptated, round, and water-dense lesion. But in case of a surgical program due to a symptomatic disease, MRI is recommended to exclude communication with the biliary tree [14].
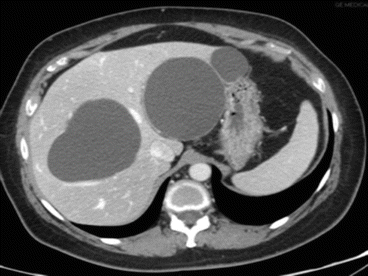
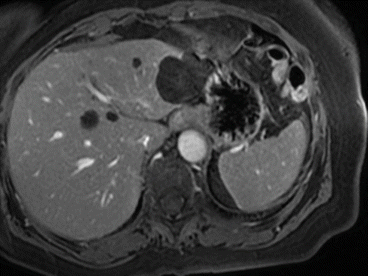

Fig. 8.8
Simple cysts at CT scan

Fig. 8.9
Simple cysts at MRI
8.2.4 Treatment
Symptomatic patients require therapy, either sclerotherapy or surgical fenestration .
8.2.4.1 Sclerotherapy
This treatment consists of the percutaneous destruction of the epithelial lining of the inner surface of the wall to disrupt the intracystic fluid secretion.
The primary indication for sclerotherapy, due to high recurrence rates, is represented by those patients who are not eligible for surgery and general anesthesia [15].
A drainage catheter, under local anesthesia, is introduced under ultrasound guidance, followed by injection of water-soluble contrast to rule out communication with adjacent bile duct or peritoneal cavity; then a sclerosing agent (ethanol, minocycline hydrochloride, and ethanolamine oleate) is injected.
The risk of alcohol intoxication limits the amount of injection to 100–200 ml [16] than, the solution is aspirated and the catheter removed.
The size of the cyst does not play a role in the amount of sclerosing agent given because after the cyst collapses, the sclerosing agent will come into contact with the cyst inner wall. Contraindications of sclerotherapy include intracystic bleeding and fistula between the cyst and biliary tree or peritoneum. The optimal efficacy may be seen up to a year after sclerotherapy, and symptomatic recurrence rate is around 20 % after 4 months [2].
8.2.4.2 Surgery
Surgical treatment consists of fenestration (with open or laparoscopic approach) and an optional transposition of an omental patch.
Fenestration consists in the excision of the roof of the cyst in order to provide communication between the inner part of the cyst and the peritoneal cavity. Excision must attentively follow the border of the liver in order to take away the most part of the cyst.
Cysts located in segments VII and VIII have the higher recurrence rates due to anatomical position.
Morbidity is 0–15 % with no mortality and a reoperation rate of 9 % [17]. Possible, although rare, complications are hemorrhage and biliary injury.
Laparoscopy has become the procedure of choice for fenestration because it is associated with significant reduction in hospital stay, postoperative pain, and morbidity and decreased blood loss with a better quality of life in the first year after surgery, as we have been able to show in a previous study, with the possibility to avoid intraabdominal adhesions in case of repeated surgery with a lower rate of complications [18].
In our experience we performed laparoscopically from 1996 to August 2014 230 laparoscopic liver procedures of which 24 (10.4 %) were fenestration for symptomatic cysts with 0 % mortality and near to 0 % morbidity.
Totally we treated surgically 32 patients with simple cyst of which only 8 (25 %) were operated with an open approach where surgical procedures for benign simple cysts was only 3 % of all surgical liver procedures performed (32/1,029).
In the Italian Registry of Laparoscopic Liver Resection held by Aldrighetti on 1,677 laparoscopic liver resection, 43 (10.4 %) were performed for benign cystic disease including simple or complicated cysts , hydatid cysts, and cystadenomas [19].
Therefore, to limit recurrence rate, it is necessary to resect as much of the wall as possible to prevent closure of the remnant wall and re-accumulation of cyst fluid. Complete resection of the cyst is not necessary and is associated with higher complication rates.
Transposition of an omental patch to the cyst bed has been advocated as a means for lowering the recurrence rate, especially in posterolateral segments where early adhesion of the liver surface to either the diaphragm or abdominal wall may lead to refilling. These observations are however anecdotic and need to be confirmed by controlled studies.
At our knowledge there are no randomized prospective studies comparing fenestration and sclerotherapy.
8.3 Polycystic Liver Disease (PCLD )
Polycystic liver is arbitrarily defined when >20 hepatic cysts are present.
PCLD is a genetic disease responsible for the development of multiple hepatic cysts . It presents in two forms, with autosomal dominant polycystic kidney disease (AD-PKD) or only in an autosomal dominant polycystic liver disease (AL-PLD) [20].
8.3.1 Pathogenesis and Epidemiology
Both forms have an autosomal dominant transmission and similar clinical presentation. PCLD associated with ADPKD is linked with mutations in the PKD1 (short arm of chromosome 16, encoding polycystin-1) or PKD2 gene (chromosome 4, encoding polycystin-2), whereas isolated PCLD is associated with heterozygous mutation in PRKC–SH or SEC63 genes [21–26].
How the gene mutations eventually lead to liver cyst formation has not been fully elucidated. Probably, a mutation in an individual intrahepatic bile duct epithelial cell results in a clonal expansion of the mutated cell, which loses communication with the intrahepatic bile ducts [27, 28].
There are some abnormalities in cholangiocyte proliferation and apoptosis and enhanced fluid secretion that are the key factors in the pathogenesis and in the progressive expansion of hepatic cysts in PCLD : cholangiocyte proliferation, fluid secretion, and cyst expansion.
Remodelling of the extracellular matrix surrounding the cysts by the activity of metalloproteases (highly elevated in liver cyst epithelia) is necessary for cyst expansion [29].
Hepatic cyst epithelium is sensitive to the proliferative effects of estrogens and IGF-1. Hereby, estrogens act not only directly but also by promoting the synthesis and release of growth factors from the cyst epithelium [30]. For this reason pregnancy, multiparity, and use of steroids further increase the risk for severe hepatic cystic disease.
The prevalence of isolated PCLD is estimated to be 1⁄100,000, while the prevalence of liver cysts in ADPKD is 58, 85, and 94 % in subgroups of age (respectively, 15–24, 25–34, and 35–46 year) [31].
Overall prevalence is the same in gender, but female population is associated with more severe liver disease.
8.3.2 Symptoms
Almost always, cysts are small and asymptomatic.
Moreover, in the majority of patients with ADPKD , PCLD is not normally the main cause of symptoms [28].
In a minority of patients (3 %), the expansion of liver cysts causes serious invalidating abdominal symptoms [32]. Data indicate that the annual growth of polycystic livers is about 0.9–3.2 % [32].
Symptoms normally begin in the third or fourth decade of life and are due to cyst growth. Presentation in childhood is therefore rare.
When present, symptoms are related mainly to the volume of the enlarged liver rather than to the volume of a specific cyst and include abdominal distension, dyspnea, epigastric pain, early satiety, vomiting, and shortness of breath [33].
Symptoms are exacerbated when there is compression of the portal vein, the inferior vena cava, or extrahepatic bile duct.
Some patients can suffer from malnutrition that is the main indication for liver transplantation.
Complications are infrequent and include bleeding, rupture, and infection of cysts [2].
The most severe complication is bacterial infection, especially in those who are under dialysis for ADPKD or in immunosuppressed patients after renal transplantation [2, 34]. The management consists of aspiration and drainage and antibiotic therapy. Cholestasis secondary to compression of adjacent biliary and portal hypertension secondary to portal or hepatic veins compression are rare.
Some patients may suffer from a rare complication as pruritus due to biliary obstruction even in the presence of normal laboratory tests [36].
Cerebrovascular pathology and cardiac-associated pathology (mitral valve prolapsed) occur respectively in 8 and 25 % of cases [37].
8.3.3 Instrumental and Laboratory Diagnosis
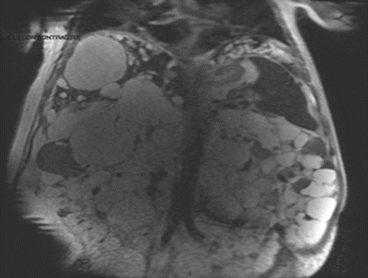
US shows multiple, fluid-filled, round or oval cysts with sharp margins being responsible for an extreme difficulty, by this tool, to identify vascular and biliary structures adjacent to the cysts. CT scan (Fig. 8.10) shows fluid attenuation with no contrast enhancement, while MRI demonstrates hyperintense on T2-weighted and hypointense on T1-weighted images [2] (Fig. 8.11). Both give a precise map of the liver and are mandatory to carry on a surgical approach.
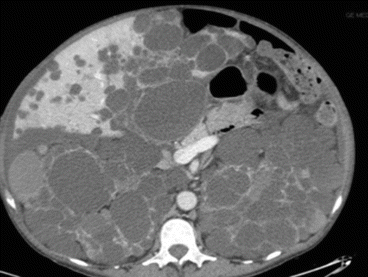

Fig. 8.10
APKD CT scan
A gastroscopy also may be useful to show a compression of the stomach or the duodenum causing early satiety, nausea, abdominal pain, and discomfort.
It is possible to stage the disease on CT scan findings according to Gigot’s classification [35] (Table 8.2) and Qian’s classification [34] (Table 8.3), while Schnelldorfer’s classification differentiates patients who could benefit from resection compared with transplantation [38] (Table 8.4).
Table 8.2
Gigot’s classification of PCLD
CT findings | Treatment | |
|---|---|---|
Type I | Less than 10 large cysts | Laparoscopic fenestration |
Type II | Diffuse involvement of liver parenchyma but with remaining large areas of non-cystic liver parenchyma | Laparoscopic fenestration |
Type III | Massive, diffuse involvement of liver parenchyma with only a few areas of normal tissue between cysts | Contraindication to fenestration requiring resection or liver transplantation in symptomatic cases
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|