Chapter 21 Intraoperative diagnostic techniques
Overview
Intraoperative ultrasonography (IOUS) continues to be an important component for assessing hepatic and biliary anatomy, for identifying and classifying malignant and cystic neoplasms, and for determining the resectability of malignant disease. Similarly, intraoperative cholangiography (IOC) assists the surgeon in assessing the biliary tree, its anatomy, and the occurrence of injury in both open and laparoscopic procedures. Diagnostic and staging laparoscopy (Table 21.1) have also emerged as important techniques for uncovering small-volume disease not revealed by even the most sophisticated imaging studies; however, controversy still exists regarding the true added value of the procedure (Table 21.2). This chapter will focus on these three intraoperative diagnostic modalities and their use in evaluating and managing hepatobiliary and pancreatic disease.
Table 21.1 Benefits of Laparoscopic Staging
Table 21.2 Potential Disadvantages of Staging Laparoscopy
Intraoperative Ultrasonography
IOUS is commonly used during both open and laparoscopic procedures. Particular attention must be given to port placement when IOUS will be performed laparoscopically. Ultrasonography (US) is most useful for evaluating the liver parenchyma or for assessing the extent of mesenteric vasculature involvement of pancreatic tumors (Jakimowicz, 1993). Other common applications include localization of pancreatic endocrine tumors, assessment of biliary calculi, and ablation of hepatic tumors.
Hepatic Disease
Evaluation of the Liver
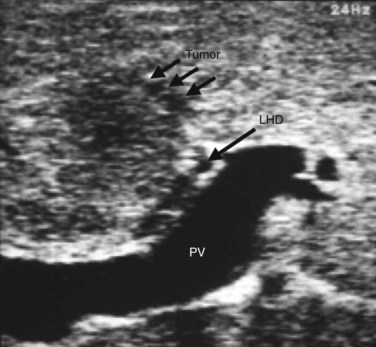
IOUS of the liver was first introduced into clinical practice in the early 1980s and rapidly became routine practice for management of malignant liver disease (Makuuchi et al, 1981; Nagasue et al, 1984; Gunven et al, 1985; Bismuth & Castaing, 1985; Belghiti et al, 1986; Gozzetti et al, 1986; Olsen, 1990; Gouillat et al, 1992; Solomon et al, 1992; Bloed et al, 2000). These early reports demonstrated that IOUS frequently altered the course of operative management of malignant hepatic tumors by more precisely delineating the relationship between tumors and major vascular and biliary structures and by detecting hepatic tumors not revealed on preoperative imaging studies (Fig. 21.1). In an analysis of 210 patients with primary and secondary hepatic malignancies, Bismuth and colleagues (1987) reported that IOUS provided additional information in 35% of cases and changed the operative plan in 20%. Parker and associates (1989) reported similar findings, with US influencing 49% of cases. A more recent analysis by Kruskal and Kane (2006) demonstrated that IOUS routinely identifies 25% to 35% more hepatic lesions than preoperative imaging studies.
It must be recognized that the yield of IOUS, particularly for identifying lesions not previously seen, depends critically on the quality of the preoperative imaging studies (see Chapters 13, 16, and 17). The results of these and other studies demonstrate the continued importance of IOUS in liver surgery for identifying and staging malignant disease.
IOUS is particularly useful for determining tumor resectability. Several reports from the 1990s have documented increased resectability rates in patients with hepatobiliary malignancy (Soyer et al, 1993; Lo et al, 1998; Jarnagin et al, 1999). More recent studies estimate that the use of IOUS will lead to changes in 15% to 25% of procedures. Regardless of these estimates, IOUS is a crucial component of the intraoperative assessment and is a useful tool in the armamentarium of liver surgeons (Zacherl et al, 2002).
Recognition of malignant disease can be challenging without the assistance of trained radiologists. Hemangiomas are typically soft and usually lack any visible flow, or they demonstrate only minimal flow compared with adjacent liver parenchyma. Metastases from colon cancer are usually hyperechoic or isoechoic to adjacent liver parenchyma and are frequently surrounded by an ill-defined hypoechoic rim that may have a bull’s-eye or target appearance. Mucinous colorectal cancer metastases may contain calcification that produces acoustic shadowing. Hepatocellular carcinoma (HCC) frequently invades major vascular structures and may be associated with porta hepatis lymphadenopathy (Jeffers et al, 1988). Documentation of lesions and their proximity to adjacent vessels will determine resectability. Resection margins of approximately 1 cm are generally sought and appear to be required for an oncologically adequate operation, although the optimal margin size is poorly defined. Keep in mind that an outline of the planned surgical margin with electrocautery of the liver capsule will produce acoustic shadowing and should be reserved until sonography is complete.
Biliary Disease
Evaluation of the Biliary Tree
As laparoscopic cholecystectomy has become the standard of care for gallbladder disease, the use of laparoscopic US during this procedure will emerge as an increasing possibility and a viable alternative to cholangiogram. Laparascopic US is now recommended as the primary screening modality for evaluating bile duct calculi because of its safety, efficiency, and overall cost effectiveness (Machi et al, 1999). IOUS is at least as good as cholangiogram, perhaps better, at detecting stones during laparoscopic cholecystectomy (Catheline et al, 1999; Siperstein et al, 1999; Tranter & Thompson, 2003). Perry and associates recently published their results from a prospective study performed between 1995 and 2005. Laparoscopic US was performed in 236 (60%) of 396 laparoscopic cholecystectomies performed for cholelithiasis. US had a positive predictive value of 100%, a negative predictive value of 99.6%, a sensitivity of 92.3%, and a specificity of 100% for detecting CBD stones (Perry et al, 2008). Proficiency with IOUS requires some time to develop, which may explain why it is not employed more liberally for biliary disease.
Laparoscopic US is a useful adjunct for oncologic staging of gallbladder carcinoma and cholangiocarcinoma. Sonographic findings not only reveal subtle liver metastases but may help to further define the local extent of invasion of these tumors into the adjacent liver bed and also outline the involvement of the ductal system, and Doppler and color flow images may help distinguish biliary sludge from polyps and other intraluminal tumors. Other applications of laparoscopic US include evaluating biliary strictures and malignant biliary obstructions and planning of surgical reconstructions such as biliary bypass procedures. These approaches and others are summarized in a review by Berber and Siperstein (2004).
Pancreatic Disease
Evaluation of the Pancreas
Other applications for US in pancreatic surgery include localizing islet cell tumors. These particularly small lesions may be difficult to visualize or palpate in the body or tail, and sonographic images may help delineate these and other malignant or premalignant tumors in the distal portions of the pancreas (Hayashibe et al, 2005). Finally, US can help identify pancreatic ductal abnormalities among patients with pancreatitis and may be useful during drainage procedures, such as cystogastrostomy.
Intraoperative Cholangiography
Technical Considerations
Intraoperative cholangiography (IOC) is most commonly used during elective cholecystectomy to assess for retained stones and to provide clarification of the biliary anatomy; rarely is it necessary or helpful in assessing extent of biliary tumors or during hepatic resection (Sotiropoulos et al, 2004). Interestingly, Mirizzi first described the procedure in 1937, as a method to help delineate the anatomy of the biliary tree in advanced cases of biliary disease (Mirizzi, 1937).
IOC is typically performed by first identifying the cystic duct at its junction with the gallbladder neck (Yamakawa, 1976). The gallbladder is retracted laterally, and the cystic duct and artery are dissected free and cleared of the fat and overlying peritoneum in the area of the triangle of Calot. A small ductotomy (less than 50% of the duct circumference) is made in the cystic duct adjacent to the gallbladder neck. The cystic duct is best approached from a right subcostal port or from the periumbilical port; we prefer the former. A 60-cm, tapered 5-Fr cholangio catheter is then advanced directly into the cystic duct through the ductotomy. A specialized cholangiogram clamp, often termed an Olsen cholangiogram clamp, secures the catheter in place (Decker et al, 2003).
Choledocholithiasis
Two factors must be considered when performing intraoperative cholangiography for the evaluation of retained bile duct stones (Hyser et al, 1999; Sigel et al, 1983; Tokumura et al, 2002). First, most stones in the biliary tree are suspected on clinical grounds or after serologic testing for liver enzyme elevation and are often visualized during preoperative US (see Chapter 13), ERCP (see Chapter 18), or MRCP (see Chapter 17). Common presentations include obstructive jaundice, cholangitis, biliary colic with abnormal liver function test results, and acute pancreatitis. The incidence of clinically silent choledocholithiasis is low, occurring in roughly one in 25 cases of biliary colic (Metcalfe et al, 2004; Nugent et al, 2005). Even when missed during cholecystectomy, these residual stones rarely if ever cause symptoms or become clinically relevant (Sarli et al, 2003a; see Chapters 30 and 35). Charfare and Cheslyn-Curtis (2003) investigated the incidence of retained biliary stones after 600 laparoscopic cholecystectomies. With a median follow-up of 3 years among 438 patients (73%), findings from this study indicate that residual bile duct stones occurred in only seven cases (1.2%).
The second factor to consider when performing IOC is that false-positive results are not uncommon, occurring up to 4% of the time (Metcalfe et al, 2004). As a result, routine application of IOC for the evaluation of unsuspected choledocholithiasis may subject patients to unnecessary bile duct explorations or ERCP studies, with considerable costs to both patients and providers (Cuschieri & Berci, 1984; Sarli et al, 2003b).
Biliary Injuries
In the late 1980s, IOC emerged as a method that could potentially help avoid major injury to the biliary system. Today laparoscopic cholecystectomy is the most commonly performed elective abdominal surgical procedure in the United States (see Chapter 34), with more than 750,000 cases performed annually. Injury to the bile duct is rare but is a major contributor to patient morbidity (see Chapter 38). These injuries are typically due to either technical error or misidentification of the duct: the technical error is that the CBD is inadvertently transected, and a surgical clip is sometimes applied to a misidentified CBD.
Four mechanisms of injury have been described (Way et al, 2003). Class I injuries involve an incision (i.e., an incomplete transection) of the CBD and typically occur when the bile duct is mistaken for a cystic duct. Class II injuries represent lateral damage to the common hepatic duct. Class III injuries are the most common and represent a CBD mistaken for a cystic duct; these are not immediately recognized intraoperatively. Finally, class IV injuries occur when a right hepatic duct is mistaken for a cystic duct.
Flum and colleagues (2003) analyzed nationwide Medicare data from 1992 to 1999. Among 1.6 million cholecystectomies performed, roughly 8000 bile duct injuries occurred overall (0.5%); these occurred in 0.39% of patients undergoing cholecystectomy with intraoperative cholangiogram and in 0.58% of patients undergoing cholecystectomy without cholangiogram (P < .001). The adjusted relative risk of bile duct injury when cholangiogram was not employed was 1.71 (95%, confidence interval [CI], 1.38 to 2.28). Inexperienced surgeons were twice as likely to cause bile duct injuries when cholangiogram was not used, as compared with their more experienced colleagues. These estimates had been observed in an earlier study (Fletcher et al, 1999).
Another benefit of intraoperative cholangiogram, with respect to biliary injuries, is the earlier recognition and correction of biliary injury (see Chapters 42A and 42B). Ideally, IOC should be performed prior to dividing the presumed cystic duct. If cannulation of the CBD, rather than the cystic duct, occurs, the cholangiogram catheter can be removed, and the ductotomy can be addressed by placing a T-tube, without the need for formal biliary reconstruction. The T-tube enables the CBD to heal without stricture formation, and the tube can be removed nonoperatively several weeks after the cholecystectomy. The alternative scenario is complete transection of the CBD, a class III injury. In the absence of cholangiogram, this transection is seldom recognized intraoperatively, typically presents postoperatively, and results in significant patient morbidity. Management undoubtedly requires an open bilioenteric anastomosis for repair, thus intraoperative cholangiogram has the potential to not only prevent bile duct injury but also to mitigate its impact if injury does occur (Giurgiu et al, 1999).