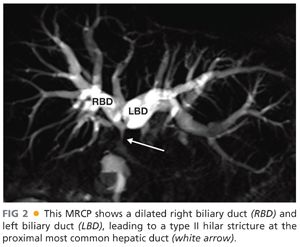
■ MRI/MRCP gives a detailed view of the biliary tract and the level or type of obstruction when a stricture is the cause (FIG 2). The vascular phases allow assessment of hepatic artery and portal vein involvement in the case of malignant processes and whether the hepatic arteries are intact and patent in cases of benign strictures related to prior biliary surgery. Finally, it allows for the assessment of direct hepatic involvement or metastatic disease.
■ When contrast for MRI cannot be given or the vascular anatomy is not adequately assessed, B-mode and Doppler ultrasonography (US) is highly accurate in assessing vascular patency and/or involvement.1,2 It is important to note whether lobar atrophy is present, as this dictates treatment options.
■ ERC and PTC are supplementary techniques for defining the anatomy of the biliary tract and the extent of a stricture when these are not clear from the MRCP. Either or both may be used to obtain a tissue diagnosis in unresectable patients or for stent placement to treat symptoms and/or for preoperative preparation. There is considerable debate as to which technique is optimal for treating hilar biliary strictures and this discussion is beyond the scope of this chapter.
■ To effectively relieve jaundice, approximately 30% of the functioning liver volume must be drained.3 The functioning and proposed drained volumes can be estimated using calculated liver volumes from preoperative cross-sectional imaging (computed tomography [CT] or MRI).4 Conversely, drainage of an atrophied hepatic lobe or segment is not effective.
SURGICAL MANAGEMENT
■ Issues associated with repair of benign strictures are discussed separately from malignant strictures.
■ Intrahepatic biliary-enteric anastomoses are complex procedures that require experienced, multidisciplinary, hepatobiliary team care. If this is not available at your institution, the patient should be referred to an appropriate institution for definitive repair.
■ All procedures are done with 2.5× magnification using loupes. This allows for meticulous surgical technique and allows precise dissection and reconstruction.
■ It is important to establish a standardize approach for biliary-enteric anastomoses. Use this each time and the high intrahepatic anastomosis will be easier to perform.
Preoperative Planning
Benign Stricture
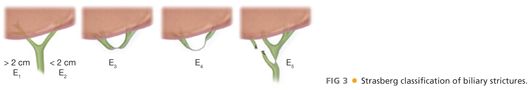
■ The goal for benign strictures is definitive restoration of biliary-enteric continuity. The type of stricture will dictate the nature of the biliary-enteric anastomosis (FIG 3).5 Types E1 to E3 strictures are amenable to a Hepp-Couinaud approach.6 E4 and E5 strictures require a modified Hepp-Couinaud approach and generally two separate anastomoses to reconstruct the right and left ducts separately.7
■ Prior to undertaking surgery, sepsis and biliary fistulae must be resolved and all the biliary segments drained. Biliary drainage usually requires a PTC for hilar (high) strictures or injuries.
■ In the clinical setting of sepsis, operative repair should not be undertaken until a minimum of 6 to 12 weeks have passed. This waiting period allows for resolution of active inflammation and evolution of any ischemic injury. Both of these are critical issues, as repair prior to resolution of these processes will decrease the success of the repair.
■ Prior to operative repair, each isolated segment of the biliary system ideally should have a PTC placed. Just prior to surgical repair, the PTC drain(s) is exchanged such that the tip is guided into the distal most part of the intubated duct(s) to intraoperatively facilitate localization.
Malignant Stricture
■ For this group, the preoperative evaluation focuses on whether the patient is a candidate for potentially curative resection. If so, they are approached as outlined in Chapter 11.
■ With unresectable disease, current percutaneous and endoscopic techniques (drainage with plastic or expandable metallic stents and intraductal photodynamic therapy or radiofrequency ablation) provide excellent palliation; thus, there is little to no role for a planned palliative intrahepatic biliary-enteric bypass.
■ Consequently, intrahepatic biliary-enteric anastomosis generally is reserved for patients with biliary obstruction found to be unresectable at the time of exploration and who are expected to survive more than 6 months. Otherwise, the morbidity and mortality associated with bypass is not justified. For instance, patients with unresectable gallbladder carcinoma have a median survival of 20 weeks. Thus, stenting is a better choice for palliation. On the other hand, the median survival for unresectable hilar cholangiocarcinoma is 52 weeks and a bypass may be reasonable under these circumstances.8 Even this circumstance is questionable, as there appears to be no survival advantage between surgical and nonsurgical drainage approaches and the morbidity and mortality for surgical approaches are significant in comparison.9
■ Other intrahepatic biliary-enteric anastomotic approaches are not included in this discussion as they are primarily of historical interest. These include the Longmire approach, mucosal graft operation, and right duct approaches. Current alternatives provide better palliation with lower risks.
■ This discussion, therefore, is limited to the segment 3 bypass (round ligament or ligamentum teres approach) as the primary practical option for these patients. Although a right sectorial duct bypass can be done, the circumstances where it might be applicable are quite limited and the results relatively poor.8 As such, it has little practical value and the best palliation for these patients is stenting and possibly tumor ablation to prevent liver failure and cholangitis.
■ Patients eligible for a segment 3 bypass are limited to those with unresectable Bismuth types I, II, or IIIa strictures without atrophy of the left hepatic lobe; to be effective, at least 30% of the functioning liver volume must be drained. Types IIIb and IV lesions are not effectively drained by a segment 3 approach. Importantly, there is no role for this bypass if the right ductal system has been contaminated by prior right duct intubation, as these patients will require continued right duct drainage to prevent cholangitis.
Positioning
■ The patient is placed in the supine position with both arms extended (FIG 4).
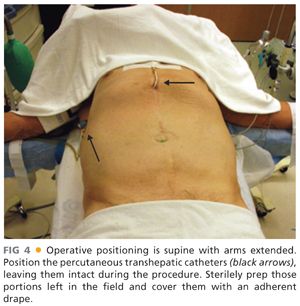
■ The percutaneous drains are aseptically prepped but positioned out of the field as much as possible (i.e., under additional sterile draping) and ideally left to drain. The catheters will need freedom of movement from within the abdominal cavity during the procedure, thus, extreme care must be taken to ensure that this is feasible without undue risk of inadvertent dislodgement of the drains.
TECHNIQUES
HILAR HEPATICOJEJUNOSTOMY (HEPP-COUINAUD APPROACH)
Preparation
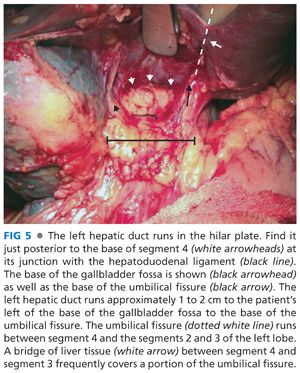
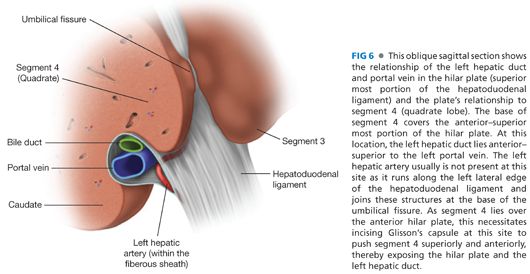
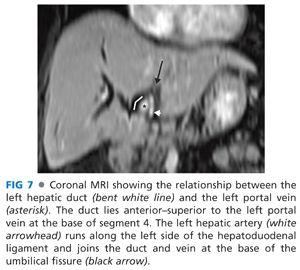
■ This approach takes advantage of the extrahepatic course of the left hepatic duct as it runs along the base of segment 4 for approximately 2 to 3 cm from the biliary confluence to the umbilical fissure (FIG 5). It is the anterior–superior most structure in the hilum, and this position facilitates its exposure and the anastomosis while minimizing injury to the portal vein and hepatic artery (FIGS 6 and 7).
Incision
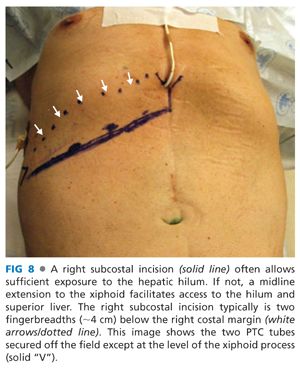
■ A right subcostal incision allows access to the hepatic hilum. Often, a midline extension facilitates full exposure (FIG 8).
Exposure
■ Identify, doubly clamp, and divide the falciform ligament. Ligate each end and leave a long tie on the superior side to use as a handle. Divide the falciform ligament superiorly to the superior edge (diaphragm) of the liver. When the patient has extensive adhesions, the ligamentum teres is an invaluable guide to locating the umbilical fissure. During dissection, follow it posteriorly to locate the umbilical fissure; this assists in identifying the anterior surface of the hepatoduodenal ligament.
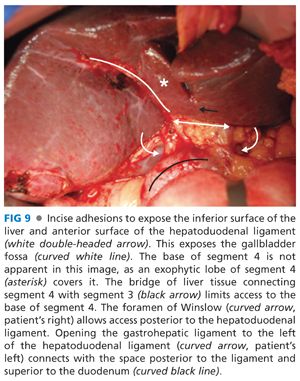
■ Take down adhesions to the inferior surface of the liver and the anterior surface of the hepatoduodenal ligament to expose the gallbladder fossa and the base of segment 4 (FIG 9). It is easiest to work from the right edge of the liver back toward the hilum when taking down adhesions. There often is a free space at the right edge of the liver that allows access posteriorly around the lateral edge of the adhesions.
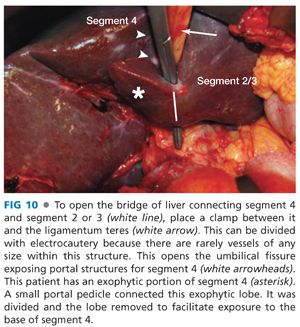
■ Place a clamp behind the usually present liver bridge joining segments 4 and 2 or 3, and divide it with electrocautery. This exposes the umbilical fissure and facilitates exposure of the left hepatic duct (FIG 10).
■ Carry the dissection to the base (posterior aspect) of segment 4 and its junction with the hepatoduodenal ligament (FIG 5). Retract the base of segment 4 superiorly and anteriorly with a malleable retractor if necessary for exposure.
■ Caution is warranted if the patient has right hepatic lobe atrophy and left lobe hypertrophy. The hepatoduodenal ligament anatomy often is distorted with the bile duct complex and hepatic arteries rotated to the right and posteriorly. Consequently, the main portal vein often is the anterior-most structure in the ligament in this situation.
Lower the Hilar Plate
■ Incise Glisson’s capsule at its junction with the hepatoduodenal ligament at the base of segment 4. This leads to entry into the hepatic parenchyma adjacent to the hilar plate (FIG 11). Venous bleeding will occur; it is easily controlled with pressure. Once the base of segment 4 is released, it is retracted further superiorly and anteriorly, exposing the hilar plate and providing access to the left hepatic duct (FIG 11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








