Sender Herschorn, MDCM, FRCSC
History of Injectable Agents
The quest for injectable agents for stress urinary incontinence (SUI) started at the end of the 19th century when Gersuny (1900) from Vienna suggested periurethral paraffin injection for urethral compression. In 1914, Kelly and Dumm warned about the dangers of embolism after injection and pointed out that this treatment showed only temporary improvement of symptoms. In 1938, Murless first reported the injection of sodium morrhuate, a sclerosing agent synthesized from cod liver oil, into the anterior vaginal wall for treatment of SUI. Of the 20 patients treated, 17 were cured or improved for at least 12 months. Sloughing of a segment of the anterior vaginal wall was seen in 12 patients, and of these 75% were cured. Murless postulated that success was due to contraction of the resulting scar of the anterior vaginal wall. Quackels (1955) reported on the injection of paraffin for incontinence after prostatectomy in 1955; and Sachse (1963), based on previous reports, injected a mineral oil preparation, granugenol oil (Dondren), another sclerosing agent. He reported cures in 12 of 24 men after prostatectomy and 4 of 7 women. However, significant complications of pulmonary emboli and urethral sloughing were seen. With the last case report of distal ureteral stenosis after periurethral injection it was recommended not to use sclerosing agents for incontinence (Bubanz et al, 1980).
Polytetrafluoroethylene (Teflon) paste was first introduced by Berg (1973) and then popularized by Politano and colleagues (1973) in the 1970s. Shortliffe and coworkers (1989) published the first report on glutaraldehyde cross-linked collagen, and Cervigni and colleagues (1994) described the use of autologous fat in woman with SUI. More recently, newer synthetic materials have been described that theoretically should improve efficacy, durability, and safety.
The ideal injectable agent should be easily injectable and conserve its volume over time. It should also be biocompatible, nonantigenic, noncarcinogenic, and nonmigratory and cause little or no inflammatory reaction (Kershen and Atala, 1999) or fibrotic ingrowth (Dmochowski and Appell, 2000). The components of the bulking agent should not separate or dissociate on injection, and if the agent contains microcrystalline or micropolymeric components they should be reasonably uniform spheres of particle sizes above 110 µm that are nonfragile and adhere to host tissue (Dmochowski and Appell, 2000). If the substance used is not successful it should not interfere with subsequent surgical intervention. To date, no substance has met all of these requirements.
Over the past 15 years there has been an evolution of injectable agents and diverse types have been tested. Table 74–1 is a list of the agents that are discussed here. Most of the agents are bulking agents, but recently injection of autologous muscle stem cells for sphincter enhancement and implantable balloons that compress the urethra have been introduced.
Table 74–1 Types of Injectable Agents for Stress Urinary Incontinence
Use of Injectable Agents in Female Stress Urinary Incontinence
Pathophysiology of Stress Urinary Incontinence and the Role of Injectable Agents
Please see the online edition of this chapter on the Expert Consult website for a discussion of this topic, along with Figures 74-1 and 74-2.![]()
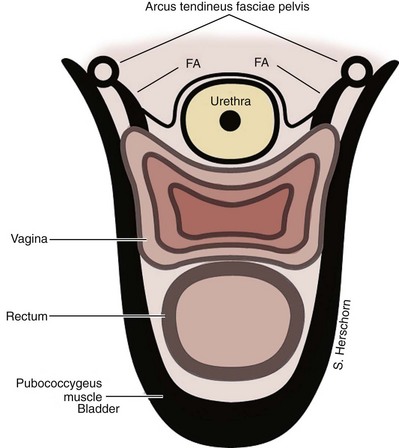
SUI is thought to be achieved by the interplay of supportive or musculofascial elements and urethral and periurethral functional elements. The anterior vaginal wall supports the urethra by its lateral attachment to the levators (pubococcygeus) and to the endopelvic fascia from the arcus tendineus fasciae pelvis (ATFP) (Fig. 74–1). This has been termed the hammock hypothesis (DeLancey, 1994). In essence it is a “double hammock” that includes not only the vaginal wall but also the posterior aspect of the levator muscles (Fig. 74–2). The lateral vaginal sulci, most clearly found in nulliparous women, at the junction of the lower and middle third of the vaginal wall, represent lateral aspects of the “hammock.” Portions of the pubococcygeus muscle are thought to attach to these sulci within the pelvis and produce elevation during voluntary contraction (Koelbl et al, 2009).
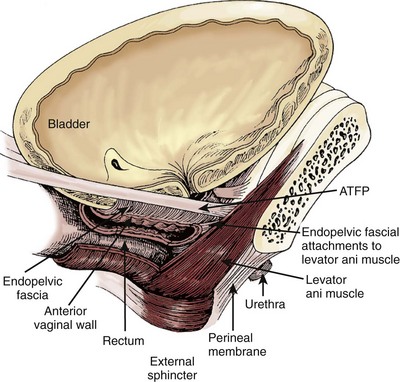
(From DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 1994;170:1713–23.)
The pubourethral ligaments are connective tissue structures that extend from the urethra to the pubic bone (Zacharin, 1963). They are sufficiently condensed to form distinct visible structures on either side of the pubis. Although they form one continuous complex they are divided into anterior and posterior pubourethral ligaments as determined by the location of urethral attachment, either anteriorly or posteriorly (Koelbl et al, 2009). Their role in maintaining continence is discussed later.
The lower third of the vagina is oriented more vertically in the nulliparous woman, and the upper two thirds is horizontal. The uterus and upper vagina are attached to the pelvic side walls by the cardinal and uterosacral ligaments. They rest posteriorly on the levator plate, which is formed by the fusion of the iliococcygeus and the posterior fibers of the pubococcygeus muscles, both part of the pelvic diaphragm. With coughing and straining the pelvic diaphragm muscles contract and the levator hiatus shortens in the anterior direction. This contributes to maintaining a normal position of the urethra and bladder with intact “hammock” support. The ‘hammock hypothesis” suggests that vaginal wall posterior to the urethra provides a backboard against which increasing intra-abdominal forces compress the urethra (DeLancey, 1994). In patients with SUI with hypermobility, restoration of suburethral or bladder neck support can result in symptom improvement or cure. This idea is supported by findings in urethral pressure transmission studies. If the pressure transmission ratio (PTR), defined as the increment in urethral pressure during coughing as a percentage of the simultaneously recorded increment in bladder pressure, is more than 90% it is of value in predicting for the presence of SUI (Cundiff et al, 1997). After successful suspension procedures, patients experience an increase in PTR during coughing (Rosenzweig et al, 1991; Athanassopoulos et al, 1994). However, in stress-continent women the PTR can be more than 100%. This may indicate that in addition to a supported suburethral vaginal wall, active forces such as pelvic floor muscle contraction occur (Constantinou, 2009).
The urethral sphincter mechanism is commonly considered to consist of striated sphincter mass and function, urethral smooth muscle, mucosal and submucosal cushions, and pudendal innervation (Koelbl et al, 2009). The idea that sphincter weakness or intrinsic sphincter deficiency (ISD) could cause incontinence independent of vaginal weakness or urethral mobility was introduced in 1976 by McGuire (Blaivas, 1993). McGuire and colleagues (1993) also characterized SUI due to ISD as associated with abdominal leak point pressures (ALPP) of 15 to 60 cm H2O, continuous leakage, and in 75% no urethral mobility (type III). They concluded that these patients, who would likely fail standard retropubic or needle suspension procedures, would be better treated by pubovaginal slings, artificial sphincters, or injection therapy because these procedures more accurately correct the underlying pathology. They stated that determination of ALPPs with clinical grading of the severity of the incontinence may identify those patients in whom leakage is unrelated to anatomic factors but the result of poor or absent urethral closing function. Furthermore, in the original series of 125 patients, 90% of the women with hypermobility had an ALPP of 60 cm H2O or greater. This separation of the patients on the basis of low ALPP having ISD and high ALPP having hypermobility was reiterated in an accompanying editorial (Blaivas, 1993).
Subsequent studies have not supported the strict division of patients into two categories. In a subsequent publication, McGuire and coworkers (1996) described a middle-pressure group (>60 to 100 cm H2O) with features of both ISD and hypermobility. Kayigil and coworkers (1999) reported that 28% of patients evaluated for SUI had both ISD and hypermobility. Kuo (2003) reported that 39% of subjects with Valsalva leak point pressure (VLPP) less than 60 cm H2O had bladder neck descent on stress testing. Fleischmann and colleagues (2003) reported a lack of relationship between the degree of hypermobility and VLPP or pad weight test in women with SUI. VLPP measures the amount of intra-abdominal or intravesical pressure required to cause incontinence during abdominal straining and is a measure of urethral function during stress maneuvers. There is no consensus about whether it should measured from the supine baseline or the standing resting baseline and about other factors, such as catheter type, placement (vaginal, rectal, intravesical), bladder volume at which measurements are done, patient position, and type of stress maneuver done (Nygaard and Heit, 2004). The variability in results seen in these and other studies as well as the lack of prospective studies predicting outcomes on the basis of VLPP argue against the categorization of patients into separate ISD and hypermobility categories. Despite the lack of its predictability of anatomic information and uncertainties related to standardization of recording methods, low VLPP (without specified values) has been widely accepted as an indicator of ISD (Koelbl et al, 2009).
The concept of separate causes of SUI is now evolving into a continuum with the acknowledgment that many patients with hypermobility also have ISD. There is good evidence that sphincter insufficiency exists in many patients. Perucchini and coworkers (2002) showed in autopsy studies that the number and density of urethral striated muscle fibers at the bladder neck and along the dorsal wall of the urethra decline with aging. Twenty-five years ago Swash and colleagues (1985) reported on pelvic floor and sphincter denervation after childbirth. More recent studies have shown a decrease in the electrophysiologic function of the pudendal nerve (Ismael et al, 2000), the striated urethral sphincter (Takahashi et al, 2000), and the pelvic floor muscles (Gunnarsson and Mattiasson, 1999; Takahashi et al, 2000), as well as prolonged pudendal nerve terminal motor latency (Bakas et al, 2001) in women with SUI. Bladder neck funneling on ultrasonography may be an indication of urethral weakness and ISD. Huang and colleagues (2003) reported funneling at rest in 111 of 320 women with primary SUI and reported that the degree of vaginal relaxation, as well as parameters of intrinsic sphincter function including VLPP and urethral closure pressure, were worse in patients with funneling than without. Similarly, Ghoniem and colleagues (2002) reported varying degrees of funneling in 91 of 100 patients with ISD. Additionally, long-term outcome studies of correction of hypermobility have suggested that there may be more urethral weakness among patients with hypermobility than previously suspected (Koelbl et al, 2009). Recently, Digesu and colleagues (2009) reported that women in whom a modified Burch colposuspension failed had significantly decreased sphincter volume on preoperatively three-dimensional ultrasonography as compared with successfully treated patients. This implies that failures may in part be attributed to concomitant ISD.
The goal of injectable agents is to augment or restore urethral mucosal coaptation and its “hermetic seal effect” contribution to the continence mechanism (Appell and Winters, 2007). It is generally thought that these agents improve intrinsic sphincter function, although the exact mechanism has not been defined (Smith et al, 2009). Bulking agents such as collagen have been reported (McGuire and Appell, 1994; Monga et al, 1995) to augment urethral mucosa and improve coaptation and intrinsic sphincter function, as evidenced by an increase in post-treatment abdominal leak point pressure (ALPP) (Herschorn et al, 1992; Richardson et al, 1995; Winters and Appell, 1995). Bulking agents do not generally obstruct voiding after the initial post-treatment period. Monga and coworkers (1995) showed that successfully treated patients have an increased area and pressure transmission ratio in the first fourth of the urethra. They suggested that placement of the injectable agent at the bladder neck or proximal urethra prevents bladder neck opening under stress, although this is controversial. Proper placement of the injectable agent, possibly just below the bladder neck, rather than actual quantity (Khullar et al, 1997) of the agent improves intrinsic sphincter deficiency (ISD).
Patient Selection, Indications, and Contraindications
Injectable agents are one of the many treatment options for SUI. Although initially it was thought that these agents would be most effective in patients with ISD alone, multiple reports have shown clinical efficacy in patients with hypermobility (Herschorn et al, 1996; Steele et al, 2000; Bent et al, 2001). Injectable agents may provide a rapid response for some patients and are an option for those who do not wish to undergo more invasive procedures. However, these patients must understand that efficacy and duration of these agents are inferior to surgery and follow-up injections may be required. Other possible indications include elderly patients, those with high anesthetic risk, or those willing to accept an improvement rather than cure of their SUI symptoms (Appell et al, 2009).
Detrusor overactivity should be treated before injection because results may be compromised (Herschorn et al, 1996). Severe urethral scarring from radiation or surgery may affect mucosal pliability by preventing bulking and retention of the injectable agent in the urethra.
Patient History
Patients who may be candidates for injectable agents should undergo a diagnostic evaluation to confirm the diagnosis in a similar fashion to other SUI patients. A focused history to characterize the chief complaint including the frequency, severity, and degree of bother should be done. An assessment of other urinary symptoms should be completed, and the desire for treatment should be ascertained. Helpful tools include various validated Symptom and Quality of Life questionnaires and frequency-volume charts (Appell et al, 2009; Abrams et al, 2010).
Pelvic Examination
The patient is placed in the lithotomy position. The external genitalia should be examined for dermatologic lesions and inflammatory conditions. The internal genitalia should be examined for estrogen deficiency, urine or abnormal vaginal discharge, pelvic organ prolapse, and abnormal pelvic masses. The poorly estrogenized vaginal wall has a thinned epithelium with loss of transverse rugae, which are normally present in its lower two thirds (Fantl et al, 1994). The patient should be examined with a comfortably full bladder to assess stress leakage and, if necessary, with an empty bladder to assess other pelvic organ prolapse and masses.
Because incontinence (or pelvic organ prolapse) may not be evident, or its full extent demonstrated, in the dorsal lithotomy position, it has been recommended that the patient be examined in the semi-upright or even upright position (Walters and Karram, 1992). A systematic examination of the vaginal walls and perineum should also be done. (For details, see Chapter 64.)
Urethral mobility can be observed with the patient straining or by the Q-tip test (Crystle et al, 1971). The angles of deflection of the Q-tip at rest and with straining are measured with a goniometer. Hypermobility is defined as a maximum strain axis of more than 30 degrees from the horizontal. Urethral axis testing does not diagnose any form of incontinence because continent women may demonstrate rotational descent of the urethra (Fantl et al, 1986) and incontinent women may have no descent. Although urethral axis testing has been shown to be reproducible (Fantl et al, 1986), it has not been compared with other radiologic methods. However, it may be helpful in assessing the degree of hypermobility.
Additional Testing
The initial evaluation of urinary incontinence in women includes a history, physical examination, urinalysis, and measurement of postvoid residual urine (Abrams et al, 2010). The basic evaluation may be satisfactory for proceeding with treatment, including surgery, for patients with straightforward SUI associated with hypermobility with normal postvoid residual volume (Fantl et al, 1996). The indications for additional testing include an inability to make a definitive diagnosis based on symptoms and the initial evaluation, concomitant overactive bladder symptoms, prior lower urinary tract surgery (including failed anti-incontinence procedures), known or suspected neurologic disease affecting the bladder, a negative stress test, abnormal findings of urinalysis such as hematuria or pyuria, abnormal levels of postvoid residual urine, beyond hymen and symptomatic pelvic prolapse, and dysfunctional voiding (Appell et al, 2009). Additional testing can include pad testing and/or use of a voiding diary, urodynamic studies, cystoscopy, and imaging. The evaluation can be tailored to elucidate the patient’s problem.
Urodynamic Studies
In the evaluation of the patient with SUI, urodynamic studies before interventional treatment are helpful in specific circumstances. These are to confirm the diagnosis if the symptoms are confusing or complex and other problems are suspected, such as detrusor overactivity, urethral obstruction or voiding dysfunction, low bladder compliance, and/or impaired or absent detrusor contractility (National Institute for Health and Clinical Excellence, 2006; Appell et al, 2009). Urodynamic studies have also been recommended if there has been previous surgery for SUI or anterior compartment prolapse (National Institute for Health and Clinical Excellence, 2006). There is controversy as to whether urodynamic studies are warranted in the straightforward or pure SUI patient with no previous surgery. The National Institute for Health and Clinical Excellence guidelines (2006), similar to the updated American Urological Association guidelines (Appell et al, 2009), did not recommend testing, whereas testing was recommended by the recent 4th International Consultation on Incontinence (Hosker et al, 2009). This was based largely on a report from Agur and coworkers (2009) that showed that only 5.2% of 6276 women in a urodynamic patient database had pure SUI and of those a fourth had other findings that could impact clinical decision making, such as detrusor overactivity, voiding dysfunction, and absence of SUI on testing.
Because injectable agents are indicated for the ISD component of SUI, can urodynamic studies assess ISD? Two measures of urethral function have been used: maximum urethral closure pressure (MUCP) and ALPP. An MUCP of less than or equal to 20 cm H2O has been suggested as indicating clinically significant urethral weakness, but there is controversy regarding the diagnostic and predictive value of urethral pressure profilometry in characterizing ISD (Weber, 2001). Similarly, an ALPP of less than or equal to 60 cm H2O was identified as an indicator of severe ISD (McGuire et al, 1993) but many studies have not confirmed the test’s value in quantifying the degree of ISD (Koelbl et al, 2009). Previously, ALPP measurements of initially less than or equal to 65 cm H2O and then less than or equal to 100 cm H2O were used as indicators of ISD to justify the use of injectable agents (Appell and Winters, 2007). However, because ISD may be present in many patients with SUI with or without urethral hypermobility (Koelbl et al, 2009), the specific value of either the MUCP or ALPP may be of little importance in the clinical decision about the use of injectable agents. As with other patients with SUI who opt for interventional therapy, urodynamic studies are helpful for the reasons mentioned earlier.
Cystoscopy
Routine cystoscopy is not recommended for the evaluation of SUI. However, it is indicated for the evaluation of incontinent patients who have sterile hematuria or pyuria; urgency incontinence to rule out other pathologic processes (e.g., bladder tumor, interstitial cystitis); recurrent or iatrogenic incontinence when surgery is indicated or planned; vesicovaginal fistula or extraurethral incontinence (Tubaro et al, 2009); and when urodynamic studies fail to duplicate symptoms of incontinence (Fantl et al, 1996). Furthermore, preinjection cystoscopy is helpful to make sure that there are no adverse factors or unexpected findings that may prevent or compromise the injection procedure such as extensive urethral scarring from previous surgery, irradiation, or trauma, foreign bodies, or urethral diverticula.
Injection Techniques
The materials can be administered under local anesthesia with cystoscopic control as an outpatient procedure. Both the periurethral and transurethral methods have been done to implant the agent within the urethral wall, preferably into the submucosa or lamina propria. It is thought that the implant should be positioned at the bladder neck or proximal urethra. Different sites can be chosen such as the 3- and 9-o’clock or 4- and 8-o’clock positions. The needle size depends on the viscosity of the agent. Preoperative and postoperative antibiotics are frequently administered. The technique of injection is seen in Figures 74-3 and 74-4. Additional bilateral periurethral infiltration of 2 to 3 mL of 1% or 2% aqueous lidocaine injected lateral to the urethra may improve patient comfort. The goal with current injectable agents is to create mucosal apposition at the end of treatment.
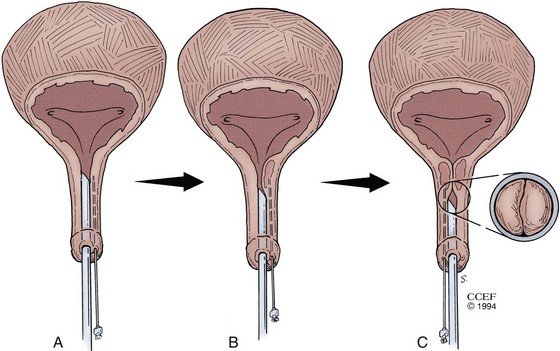
(Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1994-2011. All Rights Reserved.)
Periurethral Technique
The patient is placed in the lithotomy position and prepared and draped in the usual sterile fashion. Topical 2% urethral lidocaine jelly is instilled into the meatus. Perimeatal blebs are raised with 1% or 2% aqueous lidocaine at the 3- and 9-o’clock or 4- and 8-o’clock positions 3 to 4 mm lateral to the urethral meatus with a 25-gauge needle. A 20-Fr urethroscope with a 30-degree telescope is inserted into the urethra. The periurethral needle is introduced and advanced parallel to the endoscope sheath until its position can be seen cystoscopically just below the bladder neck within the mucosa. The surgeon can hold the cystoscope in one hand and advance the needle with the other. Care must be taken to prevent the needle from getting too close to or entering the urethral lumen because rupture of the mucosa and extravasation will occur. Rocking the needle will confirm the position of the tip. If penetration of the mucosa occurs, the needle should be removed and repositioned. The substance is injected either unilaterally or bilaterally to create the appearance of “prostatic” lobes (Fig. 74–5). Transvaginal injection with the needle placed through the biopsy port of an ultrasound probe has also been described (Appell, 1996).
An 18-gauge “bent tip needle” has been designed for the periurethral approach for placement of carbon-coated zirconium spheres (Durasphere; Boston Scientific, Natick, MA) within the proper plane (Appell and Winters, 2007).
Transurethral Techniques
With Cystoscopic Monitoring
Because of its high viscosity, silicone microimplant (Macroplastique; Uroplasty, Inc, Minnetonka, MN) injections require the use of a ratcheted injection gun (Fig. 74–6A). The injection needle is 7 Fr with a 10-mm, 18-gauge needle tip.
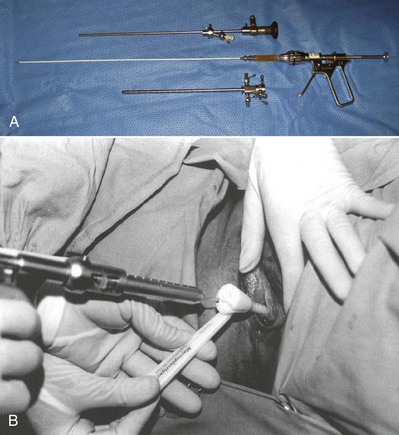
(From Tamanini JT, D’Ancona CA, Netto NR Jr. Treatment of intrinsic sphincter deficiency using the Macroplastique Implantation System: two-year follow-up. J Endourol 2004;18[9]:906–11.)
The periurethral and transurethral approaches for collagen were compared first by Faerber and colleagues (1998), who reported no significant difference in success rates and numbers of injections required in 24 patients with transurethral treatment versus 21 with a periurethral approach. However, significantly more collagen was required for the periurethral approach. Schulz and coworkers (2004) reported similar findings in 40 women randomly assigned to either technique. There was no difference in short-term success rate, but the 20 women assigned to the periurethral approach required more collagen than those assigned to the transurethral approach. The transurethral approach is now much more commonly reported than the periurethral approach.
Without Cystoscopic Monitoring
A handheld device that allows the operator to inject Macroplastique transurethrally without cystoscopy was introduced by Henalla and colleagues (2000) in a multicenter trial of 40 patients (see Fig. 74–6B). Device efficacy and acceptability were rated highly by the surgeons at 92.5% and 95%, respectively, and at 3 months 74.3% of patients had a good outcome. Twelve-month outcomes in a cohort of 21 patients who had Macroplastique injections administered with this device were reported by Tamanini and coworkers (2003): 57.1% of patients considered themselves cured, 19% were improved, and 23.8% experienced failure. Outcomes at 2 years showed some deterioration in results, with 47% considered cured, 14.3% improved, and 38.1% failed (Tamanini et al, 2004).
A handheld Implacer device was designed to administer hyaluronic acid dextranomer (Zuidex), but it was withdrawn from the market with failure of the North American multicenter randomized trial to demonstrate noninferiority of Zuidex compared with collagen (Lightner et al, 2009).
Periprocedural Care
Although randomized trials have not been done, prophylactic antibiotics with a fluoroquinolone or trimethoprim-sulfamethoxazole (TMP-SMX) for 24 hours or less can be recommended (Wolf et al, 2008). An additional 2 to 3 days has also been suggested (Appell and Winters, 2007).
Reinjections
The minimum timing for reinjections varies and depends on the agent. Although collagen can be reinjected within 7 days, most clinicians wait 4 weeks or longer to assess response of the urethra and the need for reinjection (Appell and Winters, 2007). Macroplastique injections can be repeated after 12 weeks. Durasphere can be reinjected after a minimum of 7 days (Lightner et al, 2001) and calcium hydroxyapatite (Coaptite; Bioform Medical, San Mateo, CA) can be reinjected after 1 month or less (Mayer et al, 2007). Reinjection timing with other new agents, if known, is mentioned later in the chapter.
Pitfalls in Reported Study Results of Durability
A number of pitfalls in reporting of injectable studies can lead to inflated success rates. Because injectable agents can be repeated if the patient is not a success or fails, authors should specify whether that time point is after all treatments have been completed or whether it is from baseline. If durability is reported after all injections are administered, then an accurate picture of duration of efficacy can be conveyed. A Kaplan-Meier curve of efficacy has been useful in showing what happens to patients’ continence outcome over time (Herschorn and Radomski, 1997; Lightner et al, 2001). Nevertheless, some studies report duration of results from initial treatment (Richardson et al, 1995) or do not specify it (Monga et al, 1995). This may overestimate success because failures are re-treated and can be counted as successes within the follow-up period. Another pitfall is reporting success rates on cohorts of patients followed for the long term rather than on all patients treated from the start (Stenberg et al, 2003). If the patients in whom the procedure failed or those lost to follow-up are not included in the denominator the success rate is higher.
Outcome Assessment in Bulking Agent Clinical Trials by the U.S. Food and Drug Administration
The Stamey 0 to 3 Grading System (Stamey, 1979) (Table 74–2) for SUI has been recommended as the primary outcome measure by the FDA since the original U.S. collagen trial (McGuire and Appell, 1994). Although the scale is extensively used there is little evidence that it is as valid or reliable as other measures, such as voiding diaries, pad tests, and leak point measurements (Payne et al, 2009).
Table 74–2 Stamey Incontinence Grading System
| Grade 0 | Continent |
| Grade 1 | Patient loses urine with sudden increases in abdominal pressure but not while supine |
| Grade 2 | Patient loses urine with physical stress (walking; changing from a reclining to a standing position; sitting up in bed) |
| Grade 3 | Patient with total incontinence; urine loss unrelated to physical activity and/or position |
Systematic Reviews of Injectable Agents for Women with SUI
The Cochrane Database of Systematic Reviews published an update review of injectable agents in women in 2009 (Keegan et al, 2007). The authors reviewed 12 trials with 1318 women. They concluded that the trials were small and generally of moderate quality. Pending further evidence, injection therapy may represent a useful option for short-term symptomatic relief for women with comorbidity that precludes anesthesia. Two or three injections are likely to be required to achieve a satisfactory result.
Injection therapy was also reviewed at the Fourth International Consultation on Urinary Incontinence (Smith et al, 2009). Evidence for the benefit of injectable agents was also considered to be limited and of short term. Greater symptomatic improvement was observed after surgery, which is of higher risk. There is no evidence for the superiority of any bulking agent over another (although new data on silicone microimplant injections may challenge this [Ghoniem et al, 2009]). There are also no available data comparing bulking agents with nonsurgical or minimal access surgical techniques. It was recommended that, if bulking agents are to be used, women should be made aware that repeat injections are likely to be required, that efficacy diminishes with time, and that this therapy is inferior to that of conventional surgical techniques. Women should also be aware of alternative minimally invasive procedures.
Key Points: Use of Injectable Agents in Female Stress Urinary Incontinence
Currently Used Injectable Agents
Glutaraldehyde Cross-linked Bovine Collagen (Contigen)
Glutaraldehyde cross-linked collagen (Contigen; C.R. Bard, Covington, GA) is a highly purified suspension of bovine collagen in normal saline containing at least 95% type I collagen and 1% to 5% type III collagen (Remacle et al, 1990). This cross-linking makes the collagen resistant to the fibroblast-secreted collagenase. As a result of this, the collagen is only very slightly resorbed. The implant causes little inflammatory reaction or granuloma formation and is colonized by host fibroblasts and blood vessels. It is not known to migrate; however, it does degrade over time with volume loss via absorption of the carrier medium (Kershen and Atala, 1999) and may be replaced by host collagen, to explain its persistence (Keefe et al, 1992). To decrease its antigenicity the collagen is prepared by selective hydrolysis of the nonhelicoidal amino- and carboxy-terminal groups (telopeptides) of the collagen molecule that are the antigenic parts of the molecule (Remacle and Declaye, 1988).
All patients must undergo a skin test into the volar aspect of the forearm 30 days before treatment. Approximately 3% of patients will have a positive skin test reaction, with 70% showing the reaction within 3 days, indicating a preexisting sensitivity to bovine dermal collagen through dietary exposure. The remaining 30% do not respond until later so a 4-week period is required (Keefe et al, 1992). A negative skin test does not preclude development of a hypersensitivity reaction to subsequent treatment and, although infrequently used, a second skin test has been recommended (Elson, 1989; Stothers and Goldenberg, 1998). Positive responders should be excluded.
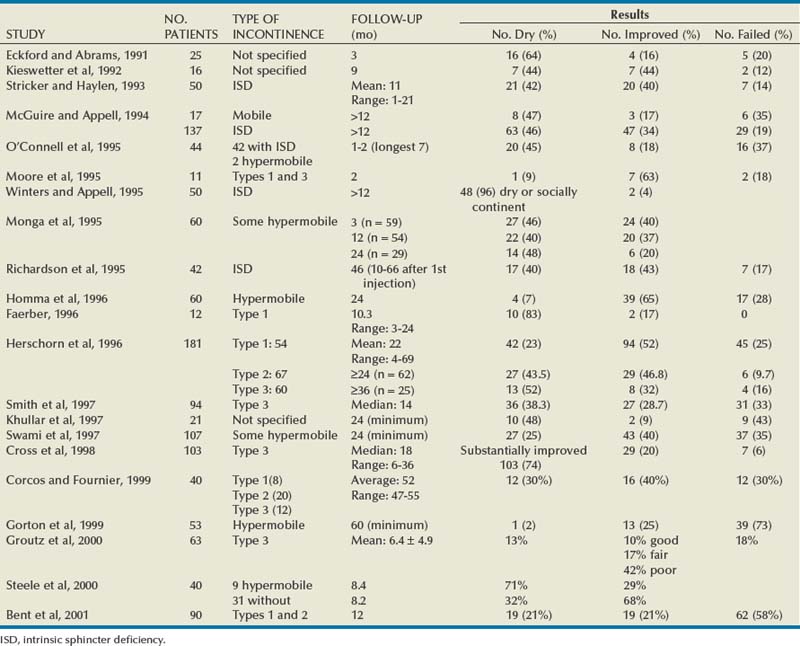
Results
The most extensively reported injectable agent has been collagen (Table 74–3). It was first reported by Shortliffe and colleagues in 1989, and since then numerous reports of its efficacy, safety, ease of administration, and relative lack of morbidity have appeared. The author’s original report, with short-term follow-up of 32 patients for 6 months (Herschorn et al, 1992), showed a cured and improved rate of 90.3%. Longer-term results of more than 1 to 2 years vary from 57%, cure and improved (Khullar et al, 1997), to 94% (Cross et al, 1998). Most patients need one to two treatment sessions with means of 5.6 to 15 mL of collagen. Winters and Appell (1995) reported a 50% rate of complete continence in a multicenter trial after 2 years. Corcos and Fournier (1999) reported 4-year follow-up with 40% improvement and 30% cure. The longest follow-up reported to date is from Gorton and coworkers (1999), who reported on 53 patients with at least 5 years after their last collagen injection: only 14 (26%) had persistent improvement, and of these 1 (2%) was completely dry. Because patients in any series are treated at different times and durations of follow-up vary, a Kaplan-Meier curve is useful to display the persistence of a good result (Herschorn and Radomski, 1997). Figure 74–7 shows that the probability of remaining dry after the last collagen injection was 72% at 1 year, 57% at 2 years, and 45% at 3 years. A similar deterioration over time was reported by Gorton and coworkers (1999).
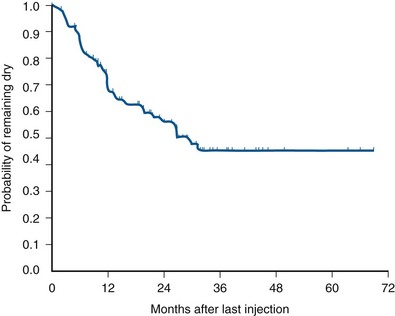
(From Herschorn S, Radomski SB. Collagen injections for genuine stress urinary incontinence: patient selection and durability. Int Urogynecol J Pelvic Floor Dysfunct 1997;8[1]:18–24.)
Multiple factors that may influence outcome have been reported. Previous incontinence surgery was identified by Eckford and Abrams (1991) as a favorable factor but was not supported by others (Herschorn et al, 1996). Preoperative detrusor overactivity may be an adverse factor (Herschorn et al, 1996; Smith et al, 1997). The degree of mucosal coaptation after injection as judged on cystoscopy was not found to correlate with long-term improvement (Kim et al, 1997). Elia and Bergman (1996) used perineal ultrasonography to measure the location of the collagen deposit 3 months after the procedure. They reported that a positive outcome was more likely if collagen was located at a distance of 6 mm or less from the bladder neck. Defreitas and coworkers (2003) correlated a good outcome to circumferential distribution of collagen around the urethra. They did three-dimensional transvaginal ultrasonography in 46 patients at a median follow-up of 14 months and found that the 21 satisfied patients had a higher rate of circumferential distribution compared with the 25 unsatisfied patients. Kuhn and colleagues (2008) compared the site of injection (i.e., bladder neck versus midurethra) in a small randomized study. They found no difference in outcome between the two sites.
Collagen and Hypermobility
The use of collagen for patients with hypermobility has been reported extensively. Moore and colleagues (1995) included patients with hypermobility. Faerber (1996) treated elderly patients with type 1 abnormality. In the report by McGuire and Appell (1994) the results at more than 1 year in women with ISD were similar to those in women with hypermobility, although there were far more women with ISD. Monga and coworkers (1995) included patients with hypermobility and found that cure rates were not reduced for women with up to 2.5 cm of movement. In the author’s series of 181 patients there was no significant difference in outcome in patients with or without hypermobility (Herschorn and Radomski, 1997). Corcos and Fournier (1999) found no difference between patients with and without bladder neck hypermobility in their 4-year follow-up on 40 patients. Steele and colleagues (2000) found that urethral mobility did not significantly affect the success rate. Furthermore, 4 of 6 patients with urethral hypermobility were dry at the 6-month follow-up examination, whereas among the 19 women without hypermobility only a 32% remained dry. Bent and colleagues (2001) reported a 44% cure/improvement rate for 12 months in a cohort of 90 women with SUI and hypermobility. They concluded, as have others, that collagen injection therapy is appropriate for women with hypermobility.
Collagen versus Surgery
Berman and Kreder (1997) found that after an average follow-up of 14.9 months after sling cystourethropexy 71.4% of the patients were continent versus 26.7% of patients with collagen after 21.3 months. They analyzed associated costs and concluded that surgery was more cost effective than collagen. In a multicenter prospective randomized trial, Corcos and colleagues (2005) reported a lower success rate of 53.1% in the collagen-treated patients versus 72.2% in the surgery group. However, general and disease-specific quality of life scores were similar, satisfaction was slightly higher in the surgery group, but complications were less frequent and severe with collagen. Corcos and colleagues concluded that collagen was a reasonable alternative to surgery. The follow-up was relatively short, and the study was done before the era of midurethral slings.
Complications
Treatment-related morbidity has been minimal. Common complications include transient urinary retention, which ranges from 1% to 21% (Herschorn et al, 1992; Winters and Appell, 1995; Appell and Winters, 2007) and can be managed with intermittent catheterization or short-term use of a Foley catheter. Urinary tract infection occurs in 1% to 25% of patients (Herschorn et al, 1992; Winters and Appell, 1995; Appell and Winters, 2007). De novo detrusor overactivity was reported in 11 of 28 elderly women (39%) treated by Khullar and coworkers (1997). Stothers and colleagues (1998) reported de novo urgency with urgency incontinence in 43 of 337 patients (12.6%), 21% of whom did not respond to anticholinergic agents. Hematuria can occur in 2% of patients (Appell and Winters, 2007). Extravasation resolves quickly with flushing away of the dilute collagen suspension and sealing over of the small needle site.
A rare complication is periurethral abscess formation (Sweat and Lightner, 1999). Another is a reaction in the previously negative skin test site after a urethral collagen injection (Stothers and Goldenberg, 1998). This occurred in 3 patients (1.9%) and was associated with arthralgias in 2. This reaction has been reported before in dermatology (Elson, 1989), and two negative pretreatment skin tests have been suggested to prevent it. The potential for hypersensitivity reactions is present because antibody production is stimulated by collagen injection (McClelland and Delustro, 1996).
Vesicovaginal fistula occurring after collagen injections for SUI in 2 women after cystectomy and neobladder was described by Pruthi and colleagues (2000). Carlin and Klutke (2000) reported a urethrovaginal fistula in a woman whose warfarin was not completely reversed. She had a postinjection hematoma that ultimately fistulized to the vagina.
Carbon-Coated Zirconium Beads (Durasphere)
Durasphere consists of nonabsorbable pyrolytic carbon-coated zirconium beads suspended in a water-based polysaccharide carrier gel of 2.8% β-glucan (Lightner et al, 2001). Pyrolytic carbon has been used in medical devices such as heart valves. The bead size ranges from 212 to 500 µm, which is larger than the threshold for particle size migration of 80 µm (Malizia et al, 1984). It is nonantigenic, and no skin testing is required. The agent can be delivered through an 18-gauge needle via the transurethral or periurethral approach with cystoscopic monitoring. It is more viscous than collagen, so greater pressure is required for delivery into the tissues.
Results
Published results with Durasphere are shown in Table 74–4. In the original multicenter randomized 235-patient clinical trial of Durasphere versus collagen, Lightner and colleagues (2001) reported a 12-month continence grade improvement rate of 76/115 (66.1%) in the Durasphere group versus 79/120 (65.8%) in the collagen group (P = 1.000). They also reported that at 1 year after their last treatment 49 of 61 (80.3%) in the Durasphere group versus 47 of 68 (69.1%) in the collagen group had a continence grade improvement (P = .162). Both injectable agents performed similarly. There was also no difference in number of injections or in results of a pad weight test. However, the injected initial and repeat injection volume of Durasphere was significantly less than those of collagen. It is notable that in the 12-month result after the last injection 45% (106/235) of the study patients were not accounted for.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree