Syndrome
Features and inheritance pattern
Gene
Familial adenomatous polyposis
Over 100 adenomatous polyps in colorectum, onset in teens
Gastric polyps
Congenital Hypertrophy of the Retinal Pigment
Epithelium
Jaw cysts
Desmoid tumours
APC
Attenuated FAP
Later onset more benign course, fewer polyps
Autosomal Dominant
Less disruptive mutations
Lynch syndrome
Microsatellite unstable tumours in colorectum, endometrium, ovary, stomach, small intestine uroepithelium, brain, skin
Autosomal dominant
Mismatch repair genes
MLH1
MSH2
MSH6
PMS2
MAP: MutYH-associated polyposis
Multiple Adenomatous colorectal polyps
Autosomal Recessive
MutYH1
Juvenile polyposis
Multiple hamartomatous polyps in colorectum
Autosomal dominant
SMAD4
Peutz jeghers syndrome
Hamartomatous polyps throughout the GI tract
Pigmentation of the mucous membranes, particularly lips
STK11
Cowden syndrome Bannayan-riley-ruvalcaba syndrome
Colonic polyps, breast cancer thyroid cancer
PTEN
Hereditary mixed polyposis
Mixed adenomatous and hamartomatous polyps in the colorectum
GREM1 (Jaeger et al. 2012)
Any of these conditions is amenable to chemoprevention strategies but most are too rare and lack clear relevance to the general population. Two conditions stand out as targets because they are well-defined and can act as a model for common cancers;
1.
Familial Adenomatous Polyposis (FAP), formerly known as Adenomatous Polyposis Coli, which results from loss of function of the APC gene on chromosome 5
2.
Lynch syndrome or Hereditary Non-Polyposis Colon Cancer (HNPCC) due to breakdown in MisMatch Repair
FAP captured clinical attention in the early twentieth century when it became clear that young people would develop hundreds, sometimes thousands, of adenomatous polyps in the colorectum leading without intervention to inevitable) death from colorectal cancer by early middle age (Burn et al.1991). Prophylactic colectomy with ileorectal anastomosis offered hope to these families where the faulty gene is transmitted as a fully penetrant autosomal dominant trait meaning that a child born to an affected parent had a 50 % chance of inheriting the faulty gene and developing the syndrome.
Systematic registries supported early gene mapping efforts and once the gene had been localised to chromosome 5q by a report of a man with FAP and learning difficulties due to a 5q deletion (Herrera et al. 1986) it took only a few months to localise and then identify the APC gene (Bodmer et al. 1987). Molecular genetic studies combined with more sophisticated clinical evaluation based on the detection of extra colonic features, particularly characteristic pigmented lesions of the retina known as congenital hypertrophy of the retinal pigment epithelium (CHRPE) resulted in highly effective family identification and more extensive endoscopic surveillance and prophylactic surgery (Burn et al. 1991).
The status of the disease was raised significantly once it became apparent that the large APC gene which harboured the hereditary defects in FAP was also usually mutated in CRC and that mutations were evident in the earliest adenomas. Somatic mutations in the APC gene are considered an initiating event in around 85 % of CRCs making FAP patients a powerful model for CRC chemoprevention studies. In essence, the cancer in an FAP gene carrier is identical to the commonest cancers in the general population. The primary difference is that in “sporadic cancer” both alleles of the APC gene must be lost in the same viable cell for neoplasia to get going where as every cell in the FAP gene carrier has one defective copy of the APC gene so only a single mutation is needed and there are millions of opportunities for that second event to occur.
There has been a long-standing interest in non-steroidal anti inflammatories in FAP. Labayle et al. (1991) reported that sulindac causes regression of rectal polyps in a randomized, placebo-controlled, double-blind crossover study in 10 patients with rectal polyps that had been previously treated by colectomy and ileorectal anastomosis. Patients received sulindac, 300 mg/day, or placebo during two 4-month periods separated by a 1-month wash-out phase. One patient was not compliant and was excluded. With sulindac, the authors observed a complete (six patients) or almost complete (three patients) regression of the polyps. With placebo, the authors observed an increase (five patients), no change (two patients), and a relative decrease (two patients) in the number of polyps. The difference between sulindac and placebo was statistically significant (p < 0.01). In biopsy specimens of polyps and normal rectal mucosa of six patients, the authors conducted an immunohistochemical study of the cellular proliferation index using the Ki 67 monoclonal antibody (Ki 67 index), at the beginning and at the end of each treatment period. They were not able to show a sulindac-induced modification of the Ki 67 index. The authors conclude that sulindac is effective in inducing the regression of rectal polyps in FAP. Giardiello et al. (1993) conducted a randomized, double-blind, placebo-controlled study of 22 patients with FAP, including 18 who had not undergone colectomy. The patients received sulindac at a dose of 150 mg orally twice a day for nine months or identical-appearing placebo tablets. The number and size of the polyps were evaluated every 3 months for 1 year. A statistically significant decrease in the mean number of polyps and their mean diameter occurred in patients treated with sulindac. When treatment was stopped at 9 months, the number of polyps had decreased to 44 % of baseline values and the diameter of the polyps to 35 % of baseline values (p = 0.014 and p < 0.001, respectively, for the comparison with the changes in the group given placebo). No patient had complete resolution of polyps. Three months after treatment with sulindac was stopped, both the number and the size of the polyps increased in sulindac-treated patients but remained significantly lower than the values at baseline. No side effects from sulindac were noted.
Nugent et al. (1993) studied sulindac in 24 FAP patients who had previously undergone prophylactic colectomy and had advanced duodenal polyposis; they were entered into a randomized trial to assess the effect of sulindac on duodenal and rectal polyps. Polyp size and number were assessed by videotaped duodenoscopy (and rectoscopy in 14 patients) at entry and after 6 months of treatment; the tapes were compared by two assessors who were unaware of the randomization and the shuffled chronological order of the recordings. Mucosal cell proliferation was measured by in vitro incorporation of 5-bromo-2′-deoxyuridine. Sulindac therapy was associated with a reduction in epithelial cell proliferation in the duodenum (median labelling index (LI) 15.8 versus 14.4 %, p = 0.003) and a trend towards duodenal polyp regression (p = 0.12). In the rectum, cell proliferation showed a marked reduction (median LI 8.5 versus 7.4 %, p = 0.018), and significant (p = 0.01) polyp regression was seen. Rectal polyposis was less severe than that in the duodenum and responded more dramatically.
A small study involving 12 FAP patients supported sulindac use (Cruz-Correa et al. 2002). Five women and seven men with men of age 37 years who had undergone ileorectal anastomosis received a mean dose of 158 mg/day for a mean period of 63.4 ± 31.3 months (range 14–98) and were followed every 4 months. Half remained polyp-free and there was a significant regression of polyps in all participants (p = 0.006). Prevention of recurrence of higher grade adenomas (tubulovillous, villous adenomas) was also observed (p = 0.004). At 35 months of follow-up, one patient developed stage III cancer in the rectal stump. The most common side effect was rectal mucosal erosions in six patients. Long-term use of sulindac was deemed to be effective in reducing polyp number and preventing recurrence of higher grade adenomas in the retained rectal segment of most FAP patients focused on the anti-inflammatory sulindac which acts as a pro drug. Metabolism in the gut converts it into the active form. They found a significant impact on polyp formation but there were some concerns about benefits in the long term.
Enthusiasm for the use of sulindac was diminished by reports of cancers emerging in rectal segments despite apparent suppression of polyp formation (Lynch et al. 1995). This may explain the disappointing results from the long-term follow-up analysis by Giardiello et al. (2002); After 4 years of treatment with either 75 or 150 mg sulindac in an RCT, 41 FAP subjects had achieved 76 % compliance. 43 % of the sulindac versus 55 % of the placebo (p = 0.54) developed polyps. There was no significant difference in polyp size or mean number.
Cyclo oxygenase exists in two forms, COX1 and COX2; the former is constitutive and its inhibition underlies the gastrointestinal toxicity of aspirin and other non-steroidals. COX2 is upregulated in inflammation prompting a search for agents which were more COX2 selective. The selective COX2 inhibitors proved to be highly effective and well tolerated leading to widespread use in the management of arthritic conditions. Recognition of the upregulation of COX2 in early colorectal cancer prompted studies of the more popular agents as cancer chemopreventives.
Steinbach et al. (2000) investigated the effects of the selective COX2 inhibtor celecoxib in FAP. In a double-blind, placebo-controlled study, they randomly assigned 77 patients to treatment with celecoxib (100 or 400 mg twice daily) or placebo for 6 months. Patients underwent endoscopy at the beginning and at the end of the study. They determined the number and size of polyps from photographs and videotapes; the response to treatment was expressed as the mean percent change from baseline.
At baseline, the mean (±SD) number of polyps in focal areas where polyps were counted was 15.5 ± 13.4 in the 15 patients assigned to placebo, 11.5 ± 8.5 in the 32 patients assigned to 100 mg of celecoxib twice a day and 12.3 ± 8.2 in the 30 patients assigned to 400 mg of celecoxib twice a day. After 6 months, the patients receiving 400 mg of celecoxib twice a day had a 28.0 % reduction in the mean number of colorectal polyps (p = 0.003 for the comparison with placebo) and a 30.7 % reduction in the polyp burden (the sum of polyp diameters) (p = 0.001), as compared with reductions of 4.5 and 4.9 %, respectively, in the placebo group. The improvement in the extent of colorectal polyposis in the group receiving 400 mg twice a day was confirmed by a panel of endoscopists who reviewed the videotapes. The reductions in the group receiving 100 mg of celecoxib twice a day were 11.9 % (p = 0.33 for the comparison with placebo) and 14.6 % (p = 0.09), respectively. The incidence of adverse events was similar among the groups. It is noteworthy that the effects were more convincing with the larger 400 mg twice daily dose which exceeds the levels at which the drug is Cox2 selective.
Huang et al. (2011) similar beneficial effects in their study of 54 celecoxib treated FAP carriers with 13 controls. The Kaplan–Meier estimated probability of not having a polypectomy 12 and 60 months post- ileorectal anastomosis in the celecoxib-treated patients (n = 33) was 60.6 and 42.2 %, respectively. The estimated probability of not having a polypectomy 6–60 months post-ileal pouch-anal anastomosis the celecoxib-treated patients (n = 24) was 100 %. The median total daily dose of celecoxib was 698.9 mg with the majority treated for more than 24 months. There was one case of a rash attributed to the treatment.
2 CAPP1
The third major randomised controlled trial in FAP was our own CAPP1 trial commenced in 1993 but was not finally published until 2011 (Burn et al. 2011a). The trial was conceived shortly after the discovery of the genetic basis and our centre had developed an active registry of FAP families, taking responsibility for management of the regular surveillance programme. It was clear that the families were keen to pursue any realistic avenue of polyp and cancer prevention. The high penetrance meant almost all gene carriers would develop disease, enhancing the statistical power of a prevention trial. The acceptance that all gene carriers should be followed by having endoscopy at least annually meant that prevention trials would be much less expensive. Funding was obtained from the European Union Concerted Action programme, giving rise to the initial name Concerted Action Polyposis Prevention. The acronym has persisted but has had a variety of meanings and in future CAPP will stand for Cancer Prevention Programme.
The CAPP1 trial focused on two interventions in a factorial design such that participants were separately randomised to each. The first agent chosen was aspirin based primarily on the early case control and observational studies suggesting its potential efficacy (Kune et al. 1988; Thun et al. 1991) and the nutritional agent, resistant starch or fermentable fibre.
Nine epidemiological studies have investigated the relationship between starch intake and CR neoplasms, with higher starch intakes providing neoplasm reductions in the range of 25–50 % (Young and Leu 2004). The Bingham team (Cassidy et al. 1994) reported a significant negative correlation between population starch intakes and colon-cancer incidence. Resistant starch (RS) is the sum of starch and the products of starch digestion not absorbed in the small intestine of healthy individuals; it undergoes colonic fermentation to short-chain fatty acids including butyrate. RS supplementation can improve a number of potential biomarkers of CRC risk including faecal concentrations of total and secondary bile acids, which it lowers (Hylla et al. 1998; Grubben et al. 2001). Butyrate and non-steroidal anti-inflammatory drugs (NSAIDs) differ radically in the transcriptome and proteome modifications they produce in tumour cells (Williams et al. 2003; van Munster et al. 1994).
CAPP1 aimed to use adenoma development in adolescents with FAP as the primary measure and effects on mucosal crypt dimensions and cell proliferation as secondary biomarkers of CRC chemoprevention. Polyposis registry clinicians across Europe recruited young male and female FAP patients between 10 and 21 years. Restricting eligibility to members of families manifesting classical FAP helped ensure homogeneity. This restriction meant exclusion of people with less damaging mutations which result in Attenuated or Atypical FAP (AFAP for short). Current NSAID therapy, known aspirin sensitivity, major intercurrent illness and pregnancy were grounds for exclusion. The eligibility cut-off at 21-years old was based on concerns that continuation beyond this age might risk delaying preventive surgery.
2.1 CAPP1 Trial Design
CAPP1 was a double-blind, randomized trial with four arms: aspirin (600 mg as 2 tablets/d) plus matched placebo, RS (30 g as 2 sachets/d in a 1:1 blend of potato starch and high amylose maize starch [Hylon VII]) plus matched placebo, aspirin plus RS, and placebo plus placebo; this trial employed a 2 × 2 factorial design. The accrual goal was 208 patients, 52 in each of the four intervention arms.
Randomization was in blocks of 16 stratified at the level of European geographical regions. The duration of intervention was from 1 to a potential maximum of 12 years, with a scheduled annual endoscopy. Patients were advised of their option to leave intervention after each annual examination but were invited to remain on intervention up to age 21 years.
The primary trial endpoint was the proportion of patients with an increased polyp count in the rectum and sigmoid colon after intervention. A major secondary endpoint was size of the largest polyp, which was chosen as another quantifiable and objective measure of disease severity. Given the multiple, international centres participating in this trial, it was not feasible to influence the clinical decision on whether the largest polyp was removed or left in situ nor to tattoo any polyps for review at a later examination.
Secondary laboratory endpoints were proliferative-state assessments assayed using three techniques; samples were microdissected to enable direct counts of total numbers (and location) of mitoses in whole crypts. Formalin-fixed biopsies were paraffin-embedded and sectioned routinely prior to immunohistochemical staining by MIB1 to detect Ki67 and by PC10 to detect PCNA (Mills et al. 2001).
2.2 CAPP1 Endpoint Ascertainments
Endoscopists counted the actual number of polyps in the rectum and sigmoid colon if there were 10 or fewer and provided an estimate (11–15, 16–20, 21–30, 31–50, 51–100, >100) when more numerous. Data were collected on the total number of polyps (rectum and sigmoid, ascending, transverse, and descending colon), which presented a challenge because of differing endoscopy policies and the challenge of the total number of polyps in an FAP patient, which can run into the thousands. The situation was made more challenging by marked variation across centres in technology used and the extent of examination. We used diagrams with count estimates, the size of the largest polyp seen and withdrawal rectal videos. The video recordings were scored (better, worse or same) by two experienced endoscopists blinded to intervention and the time point of examination.
2.3 CAPP1 Laboratory and Statistical Methods
For assessing proliferative-state endpoints six variously fixed biopsies were taken from normal mucosa near the anal verge at the baseline and annual examinations and microdissected to enable direct counts of total numbers (and location) of mitoses in whole crypts, and measurement of crypt width and length.
The study was designed to detect a statistically significant difference in the proportion of patients with an increased polyp number in the rectum and sigmoid colon for aspirin versus non-aspirin groups or in the RS versus non-RS groups. We included the secondary endpoint of the total number of polyps throughout the colon (adjusted for the extent of endoscopy to account for the variable completeness of endoscopy) so as to make more complete use of collected data. Variability in local policy over extent of endoscopy meant that polyps were counted, or estimated when too dense, in differing numbers of colorectal segments.
We estimated that 40 % of the patients in the placebo group would have an increased polyp count at the end of intervention compared to 20 % in the aspirin or RS groups based on published observational studies (Giovannucci et al. 1995). Due to the suggestion in some of these studies that the effect was greater with prolonged use, this study was designed to allow participants to remain on study for as long as possible. The final sample size estimate of 208 patients was based on early data indicating that almost all patients had detectable pathology and was designed to detect the anticipated intervention effect with an 80 % power and alpha level of 0.025.
A supplementary prespecified secondary analysis was the size of the largest polyp at the end of intervention and assessed in the subset of patients who remained on study for >1 year; these patients were anticipated to be more likely fully compliant and to respond to an active intervention.
2.4 CAPP1 Results
Initially, 227 young people aged from 10 to 21 years with intact colons were recruited into the study (1993–2002) and 206 of these started the intervention (Fig. 1). Fifty-nine percent (133/227) had a baseline and at least one other endoscopy and were therefore eligible for data analyses. (Table 2). Some patients remained on intervention for up to 7 years before the study ended.
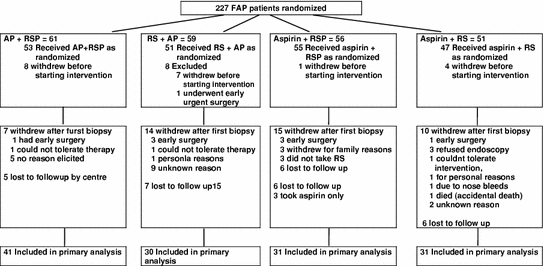

Fig. 1
CAPP1 consort diagram
Table 2
CAPP1 Patient characteristics
Baseline measures (range of values) | Number | Intervention (N)a | P-value | |||||
|---|---|---|---|---|---|---|---|---|
RSP/AP (41) | RS/AP (30) | A/RSP (31) | A/RS (31) | |||||
Age | Mean (s.d) N | 133 | 18.2 (7.8) 41 | 17.9 (10.8) 30 | 17.2 (6.9) 31 | 18.8 (7.9) 31 | 0.90 | |
Sex | N (%) | Female | 66 | 19 (46.3) | 21 (70.0) | 12 (41.4) | 14 (45.2) | Χ2(3) = 6.2, p = 0.10 |
Male | 65 | 22 (53.7) | 9 (30.0) | 17 (58.6) | 17 (54.8) | |||
Total | 131 | 41 (100) | 30 (100) | 29 (100) | 31 (100) | |||
Number of endoscopies | N | 2 | 76 | 20 | 19 | 21 | 16 | |
3 | 30 | 12 | 3 | 3 | 12 | |||
4 | 15 | 4 | 5 | 4 | 2 | |||
5 | 8 | 4 | 3 | 1 | ||||
6 | 1 | 1 | ||||||
7 | 2 | 2 | ||||||
8 | 1 | 1 | ||||||
Total | 133 | 41 | 30 | 31 | 31 | |||
Polyp data | ||||||||
At least 1 polyp found | N (%) | No | 13 | 1 (2.4) | 5 (17.9) | 3 (10.0) | 4 (13.3) | Χ2(3) = 4.8, p = 0.18 |
Yes | 116 | 40 (97.6) | 23 (82.1) | 27 (90.0) | 26 (86.7) | |||
Total | 129 | 41 (100) | 28 (100) | 30 (100) | 30 (100) | |||
Number of polyps in the rectum and sigmoid colon (0, 200) | Mean (s.d) | 29.8 (43.0) | 29.7 | 22.7 | 25.6 | 0.53b | ||
N | 129 | 41 | (52.5) 28 | (32.3) 30 | (44.4) 30 | |||
Total number of polyps (0, 425) | Mean (s.d) | 56.7 (86.4) | 63.8 | 44.5 | 43.1 | 0.60b | ||
N | 129 | 41 | (115.6) 28 | (90.5) 30 | (76.2) 30 | |||
Size of largest polyp (mm) (0.5, 50) | Mean (s.d) | 6.1 (8.2) | 4.2 (2.7) | 4.0 (2.5) | 4.3 (2.4) | 0.63b | ||
N | 110 | 38 | 21 | 27 | 24 | |||
Crypts | ||||||||
Crypt width (74.2, 199.7) | Mean (s.d) | 121.5 (21.6) | 110.7 (20.3) | 116.8 (24.1) | 111.5 (17.3) | 0.16b | ||
N | 113 | 35 | 24 | 25 | 29 | |||
Crypt length (290.4, 765.1) | Mean (s.d) | 499.4 (84.4) | 467.3 (92.9) | 494.8 (75.6) | 498.6 (69.1) | 0.27b | ||
N | 112 | 35 | 24 | 25 | 28 | |||
Mean total CCP (0.2, 37.9) | Mean (s.d) | 5.3 (4.9) | 6.1 (7.8) | 7.5 (6.1) | 6.1 (4.2) | 0.27b | ||
N | 113 | 35 | 24 | 25 | 29 | |||
Withdrawal rates in each intervention group were compatible with random loss. For the 94 patients excluded from analysis, there was a non-significant difference in age at “dropout” between the four intervention groups (ANOVA, p = 0.06). Of the patients included in the final analysis, 57 % (76/133) had two colonoscopies, 23 % (30/133) had three colonoscopies, 11 % (15/133) had four and 9 % (12/133) had 5–8 colonoscopies. No polyps were found in 15 % of the colonoscopies; 57 % of the colonoscopies went further than the sigmoid colon.
At baseline, the four intervention groups were well matched; at entry endoscopy, 41 % of patients had one or more polyps removed; these patients tended to be older.
After a median intervention period of 17 months (range 1–73), the primary endpoint of a risk of an increased polyp number in the rectum and sigmoid colon was not significantly reduced in either treatment group with relative risks of 0.77 (aspirin; 95 % CI, 0.54–1.10;) and 1.05 (RS; 95 % CI, 0.73–1.49; Table 3). The diameter of the largest polyp (major secondary endpoint) detected by the endoscopist at the end of intervention tended to be smaller in the aspirin arm (p = 0.05 and p = 0.09 after adjusting for baseline measures; Table 4. The planned subgroup analyses of patients who elected to continue on study for more than 1 year found a significant reduction in the size of the largest polyp in the aspirin versus non-aspirin group (p = 0.02, adjusted for baseline; Table 4 and Fig. 2). We found an absence of polyps in the majority of our blinded review of rectal videos (both at baseline and during intervention), even though there were adenomas in the colon making these recordings of little value. The risk of an increased total number of polyps in all examined segments of the colorectum was not reduced in either intervention group, with relative risks of 0.97 (aspirin; 95 % CI, 0.65–1.43;) and 0.96 (RS; 95 % CI, 0.65–1.42;).
Table 3




Relative risks (RelRs) and 95 % confidence intervals (CIs) from 12 univariate modelsa estimating the effect of intervention by outcome measure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







