(1)
Stroke Prevention Research Unit, Nuffield Department of Clinical Neuroscience, John Radcliffe Hospital, Oxford, OX39DU, UK
Abstract
In addition to longstanding evidence from observational studies, evidence from randomised trials of the effectiveness of aspirin for chemoprevention of colorectal cancer has increased substantially in recent years. Trials have shown that daily aspirin reduces the risk of any recurrent colorectal adenoma by 17 % and advanced adenoma by 28 %, and that daily aspirin for about 5 years reduces incidence and mortality due to colorectal cancer by 30–40 % after 20 years of follow-up, and reduces the 20-year risk of all-cause cancer mortality by about 20 %. Recent evidence also shows that the risk of major bleeding on aspirin diminishes with prolonged use, suggesting that the balance of risk and benefit favours the use of daily aspirin in primary prevention of colorectal and other cancers. Updated clinical guidelines are currently awaited.
1 Introduction
Colorectal cancer accounts for about 10 % of all incident cancers about 8 % of all cancer mortality Weitz et al. (2008) and the lifetime risk in developed countries is about 5 %. Sigmoidoscopic and endoscopic screening and polypectomy are partially effective in preventing colorectal cancer, particularly for cancers of the distal colon and rectum (Rex et al. 2009; Atkin et al. 2010), but there is still a substantial clinical need for other supplementary preventive strategies. There is good observational evidence that modifiable lifestyle and dietary factors are important in the aetiology of colorectal cancer, but there remains significant need for pharmacological chemoprevention if a safe and effective agent were available. As detailed by others in this book, aspirin is the most widely studied pharmacological agent for the prevention of colorectal cancer. However, clinical guidelines currently recommended against the routine use of aspirin for colorectal cancer prevention (Cuzick et al. 2009). The aim of this chapter is to first review the evolution of the evidence that aspirin has a clinically useful effect in long-term primary prevention of sporadic colorectal cancer in man and, second, to consider that effect within the context of other indications and effects of aspirin in prevention of vascular disease.
2 Aspirin in Prevention of Colorectal Cancer
2.1 Observational Studies
Observational studies can reliably identify powerful causal associations (e.g. smoking and lung cancer, cholesterol and coronary heart disease, blood pressure and stroke, radiation exposure and cancer, sleeping position and sudden infant death and male circumcision and incidence of HIV infection), but they have proved less reliable in studying behavioural risk factors that involve an element of choice, such as diet or use of vitamins, hormone replacement therapy). Nevertheless, much has been learned from observational studies of the association between use of aspirin and risk of colorectal cancer.
In 1988, Kune and colleagues published the first report of an inverse association between use of aspirin and the risk of colorectal cancer in man (Kune et al. 1988). They investigated associations among colorectal cancer and a variety of chronic illnesses, operations and medications in 715 patients with incident, histologically confirmed colorectal cancer and 727 age and sex-matched controls. The authors did not mention any a priori hypotheses about an effect of aspirin, but they found that patients with colorectal cancer were less likely than controls to have used aspirin-containing medications in the past (relative risk 0.53, 95 % confidence interval 0.40–0.71).9 This association remained significant after adjustment for comorbidities and was consistent for both men and women. A similar association was found for use of other NSAIDS but was confined to colon cancers only. They examined over 30 other potential associations with colorectal cancer and found that hypertension, heart disease, stroke, chronic chest disease, chronic arthritis and use of vitamins supplements were also less common amongst patients with colorectal cancer, whereas haemorrhoids and large bowel polyps were more common amongst cases. However, prior aspirin use remained inversely associated with colorectal cancer in a multivariate model that included all of the statistically significant univariate associations as well as previously identified dietary factors.
The Kune study stimulated considerable interest. A subsequent analysis of 662,424 men and women enrolled in the Cancer Prevention Study II cohort showed that aspirin use at least 16 times per month was associated with a 40 % reduced risk of colon cancer mortality over a 6-year period (Thun et al. 1991). An updated analysis of this cohort observed that daily use of at least 325 mg for at least 5 years was associated with a lower incidence of colorectal cancer compared with non-users (RR, 0.68; 95 % CI, 0.52–0.90) as well as reduced risks of other cancers (Jacobs et al. 2007). Another cohort study of 47,363 men from the Health Professionals Follow-up study showed that regular aspirin users (≥2 times/week) had a 21 % (RR, 0.79; 95 % CI, 0.69–0.90) lower risk of colorectal cancer over 18 years of follow-up (Chan et al. 2008). Similar findings were observed in a cohort of 82,911 women from the Nurses’ Health Study; regular aspirin use (≥two 325 mg tablets/week) was associated with a 23 % reduced risk of colorectal cancer (RR, 0.77; 95 % CI, 0.67–0.88) over 20 years of follow-up (Chan et al. 2005).
By 2007, data on the association between aspirin use and colorectal cancer were available from 11 cohort studies and 19 case-control studies (Flossmann and Rothwell 2007). In a meta-analysis of the case-control studies, there was significantly lower use of aspirin or NSAID in cases than in controls (pooled OR = 0.80, 95 % CI 0.73–0.87, p < 0.0001, Fig. 1a), but with substantial heterogeneity between studies (p < 0.0001) (Flossmann and Rothwell 2007). Associations tended to be much stronger in smaller studies, with a highly significantly asymmetrical funnel plot (Fig. 1a), which could be misinterpreted as evidence of publication bias and overestimation of any true effect. However, on closer scrutiny the asymmetrical funnel plot appeared to be due to more discriminating definitions of use of aspirin or NSAID in smaller studies, with a strong inverse relation (weighted regression: r 2 = 0.53, p = 0.0005) between the percentage of the control group defined as users and the relative use of aspirin in cases versus controls (Flossmann and Rothwell 2007).
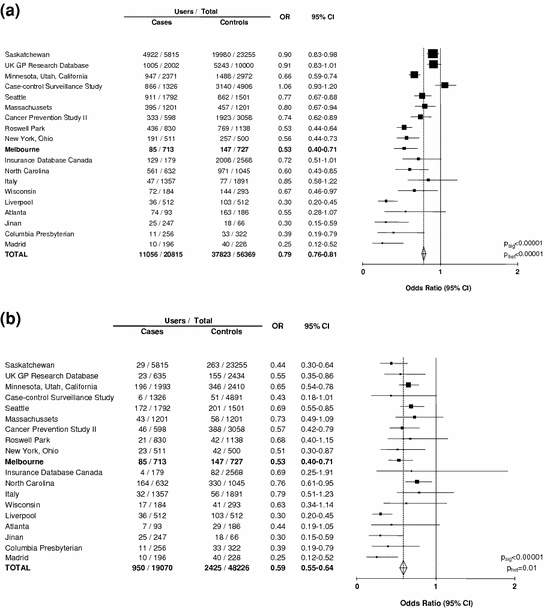

Fig. 1
a Any use of aspirin and/or NSAID in cases of colorectal cancer versus age and sex-matched controls in 19 case-control studies. (Data derived from Flossmann and Rothwell 2007). b Maximum use of aspirin and/or NSAID in cases of colorectal cancer versus age and sex-matched controls in 19 case-control studies. (Data derived from Flossmann and Rothwell 2007). The Melbourne Colorectal Cancer Study (Kune et al. 1988), the first such report, is highlighted in bold font
Fourteen studies stratified analyses by the extent of use of aspirin or NSAID, and when the analysis of all 19 studies was based on the maximum use reported (most regular and/or longest duration) the association with colorectal cancer was much stronger and less heterogeneous (Fig. 1b), and was no longer related to the percentage of controls defined as users (r 2 = 0.10, p = 0.18). In eight studies where it was possible to look specifically at irregular or occasional use of aspirin or NSAID, there was no association with colorectal cancer (OR = 1.01, 0.93–1.09, p = 0.87). Those studies that stratified analyses by both regularity of use and duration of use reported 50–70 % reductions in relative risk of colorectal cancer associated with use of medium to high dose aspirin for over 10 years (Flossmann and Rothwell 2007).
Given the considerable statistical power in these observational studies (the cohort studies included 1,136,110 individuals with over 6,000 colorectal cancers during follow-up and the 19 case-control studies included 20,815 cases of colorectal cancer), they can also be used to address clinically important questions about heterogeneity of effect. For example, the observational data demonstrated no difference in effect of aspirin and other NSAIDs, no difference in relation to age, sex, race or family history, no difference in relation to the site or aggressiveness of the cancer and no fall-off in apparent effect with use for ≥20 years (Flossmann and Rothwell 2007).
2.2 Randomised Controlled Trials
There are relatively few data from randomised trials of the effect of aspirin on risk of colorectal cancer compared with the wealth of data from observational studies. The first randomised trial of aspirin was as an adjuvant treatment in patients with established colorectal cancer in the late 1970s and early 1980s. No benefit was seen, but the trial was small and underpowered.8 Starting in the 1990s, several randomised trials (Baron et al. 2003; Benamouzig et al. 2003; Logan et al. 2008; Sandler et al., 2003) showed that aspirin reduced the recurrence of colorectal adenomas by 20–30 % in patients with previous adenomas or colorectal cancer (Table 1). Meta-analysis of the four trials of aspirin versus placebo, which together included nearly 3,000 patients, showed that aspirin at any dose (81–325 mg/day) reduced the risk of any colorectal adenoma (defined as occurrence after randomisation) by 17 % (RR = 0.83; 95 % CI, 0.72–0.96) over a median post-randomisation follow-up of 33 months (Cole et al. 2009). The risk of advanced colorectal adenomas (defined as 1 cm or larger in size or with high-grade dysplasia or invasive cancer) was reduced by 28 % (RR = 0.72; 95 % CI, 0.57–0.90).
APACC | AFPPS | CALGB | ukCAP | |
|---|---|---|---|---|
Design | Randomised controlled trial | Randomised controlled trial | Randomised controlled trial | Randomised controlled trial |
Aspirin (160 or 300 mg/day) or placebo Follow-up: 4 years | Aspirin (81 or 325 mg/day) or placebo Follow-up: 3 years | Aspirin (325 mg/day) or placebo Median follow-up: 12.8 months | Aspirin (300 mg/day) or folate supplement (0.5 mg/day) Follow-up: 3 years | |
Patients (n) | 272 | 1,121 | 635 | 945 |
Adenoma inclusion criteria | Recent history of colorectal adenomas | Recent history of colorectal adenomas | Previous history of colorectal cancers | Recent history of colorectal adenomas |
Family history of adenomas (%) | 34.6 | 30.4 | Not reported | 14.1 |
RR (95 % CI) for any adenomaa | 0.95 (0.75−1.21) | 0.88 (0.77−1.02) | 0.61 (0.44−0.86) | 0.79 (0.63−0.99) |
RR (95 % CI) for advanced adenomaa | 0.91 (0.51−1.60) | 0.74 (0.52−1.06) | 0.77 (0.29−2.05) | 0.63 (0.43−0.91) |
Since adenomas are the precursors of the majority of colorectal cancers (Morson 1984; Levine and Ahnen 2006), these effects of aspirin on risk of recurrent adenoma were encouraging, but with only 2–3 years follow-up the trials were unable to determine any effect on risk of colorectal cancer. A reduction in risk of cancer can not simply be assumed to be an inevitable consequence of the effect of aspirin on recurrence of adenomas. The likelihood of malignant transformation of adenomas that develop despite aspirin versus those that are prevented is uncertain. Although up to 40 % of people in developed countries have one or more colorectal adenomas by age 60 years, less than 10 % of these adenomas progress to cancer. Moreover, it could not be assumed that secondary prevention of adenomas by short-term treatment with aspirin would be maintained on long-term treatment, nor that the same effect would necessarily be seen in patients without a prior history of colorectal neoplasia.
It was still necessary, therefore, to obtain confirmation from randomised trials that aspirin could prevent colorectal cancer in primary prevention. However, a latency of more than 10 years would be expected prior to an effect of aspirin becoming evident, given that the delay between the initiation of development of an adenoma, the point at which aspirin is believed to act and presentation of colorectal cancer is estimated to be 10–15 years (Kozuka et al. 1975; Kelloff et al. 2004). Indeed, two large randomised trials of alternate-day aspirin in primary prevention of vascular disease had shown no effect on risk of colorectal cancer during 10 years of follow-up (Stürmer et al. 1998; Cook et al. 2005), and the results of short-term follow-up in cohort studies were similarly unpromising (Flossmann and Rothwell 2007).
Given the likely need for up to 15–20 years follow-up in order to reliably determine the effect of a period of treatment with aspirin on risk of colorectal cancer, new prospective randomised trials were unlikely to be done. It was possible, however, to follow-up patients who had been randomised in previous trials of aspirin in prevention of vascular events in the 1980 and 1990s in order to examine if any delayed effect on incidence or mortality due to colorectal cancer was evident on long-term follow-up after the trials. The first, such, study (Flossmann and Rothwell 2007) reported 20-year follow-up of two UK trials of daily high-dose aspirin versus control, the UK-TIA Aspirin Trial (1,200 vs. 300 mg vs. placebo; Farrel et al. 1991) and the British Doctors Aspirin Trial (500 mg vs. control; Peto et al. 1988). Allocation to aspirin reduced incidence of colorectal cancer (pooled HR = 0.74, 0.56–0.97, p = 0.02 overall; 0.63, 0.47–0.85, p = 0.002 if allocated aspirin for ≥5 years). However, as predicted, this effect was only seen after a latency of 10 years (0–9 years, HR = 0.92, 0.56–1.49, p = 0.73; 10–19 years, HR = 0.60, 0.42–0.87, p = 0.007) and was greatest 10–14 years after randomisation in patients who had scheduled trial treatment of ≥5 years (HR = 0.37, 0.20–0.70, p = 0.002, Table 2). The authors concluded that use of aspirin ≥300 mg daily for about 5 years was effective in primary prevention of colorectal cancer, with a latency of about 10 years.
Table 2
Hazard ratios (95 % CI) for diagnosis of colorectal cancer in a pooled analysis (stratified by trial) of data from the British doctors aspirin trial and the UK-TIA Aspirin trial of long-term follow-up after the scheduled trial treatment period, stratified into 5-year periods (Flossmann and Rothwell 2007)
Years from randomisation | All complianta patients with scheduled trial treatment of ≥5 years | All complianta patients with scheduled trial treatment of ≥5 years | ||
|---|---|---|---|---|
5–9 yearsb | 1.08 (0.55–2.14) p = 0.83 | 0.83 (0.38–1.80) p = 0.63 | 0.93 (0.42–2.09) p = 0.86 | 0.67 (0.25–1.78) p = 0.67 |
10–14 years | 0.51 (0.29–0.90) p = 0.02 | 0.43 (0.23–0.79) p = 0.007 | 0.37 (0.20–0.70) p = 0.002 | 0.26 (0.12–0.56) p = 0.0002 |
15–19 years | 0.70 (0.43–1.14) p = 0.15 | 0.67 (0.39–1.14) p = 0.14 | 0.69 (0.42–1.15) p = 0.16 | 0.66 (0.37–1.16) p = 0.15 |
≥20 years | 0.90 (0.42–1.95) p = 0.79 | 0.85 (0.36–2.03) p = 0.72 | 0.73 (0.33–1.63) p = 0.45 | 0.65 (0.26–1.63) p = 0.35 |
These data on the long-term effects of high-dose aspirin were useful proof of principle, but did not change clinical practice because of concern that the adverse effects of long-term use of high-dose aspirin would limit its potential for long-term prevention. Lower doses (75–300 mg daily) reduced the short-term risk of recurrent colorectal adenomas, but effectiveness in long-term primary prevention of colorectal cancer was unknown. Post-trial follow-up was, therefore, done (Rothwell et al. 2010) in three randomised trials of low-dose aspirin versus control, one in primary prevention of vascular events (Medical Research Council’s General Practice Research Framework 1998) and two in secondary prevention after TIA or stroke (SALT Collaborative Group 1991; Dutch TIA Trial Study Group 1991).
The effect of allocation to aspirin on the long-term risk of death due to colorectal cancer in the three trials of low-dose aspirin was very similar to that in the trials of high-dose aspirin (Fig. 2) (Rothwell et al. 2010). There was also a significant reduction in long-term incidence of colorectal cancer in patients allocated low-dose aspirin (Fig. 3). A pooled analysis of the four trials of high-dose or low-dose aspirin versus control (mean duration of scheduled treatment = 6.0 years) was, therefore, performed Rothwell et al. 2010. Among 14,033 patients, 391 developed colorectal cancer during median follow-up of 18.3 years, with allocation to aspirin reducing the risk of colon cancer (incidence–HR = 0.76, 0.60–0.96, p = 0.02; mortality–0.65, 0.48–0.88, p = 0.005), but not rectal cancer (0.90, 0.63–1.30, p = 0.58; 0.80, 0.50–1.28, p = 0.35). Where anatomic subsite data were available, aspirin reduced risk of cancer of the proximal colon (incidence–0.45, 0.28–0.74, p = 0.001; mortality–0.34, 0.18–0.66, p = 0.001), but not the distal colon (1.10, 0.73–1.64, p = 0.66; 1.21, 0.66–2.24, p = 0.54): difference–p = 0.04 for incidence, p = 0.01 for mortality (Fig. 4). Benefit increased with scheduled duration of trial treatment (Rothwell et al. 2010). After scheduled treatment for ≥5 years (Fig. 4), aspirin reduced risk of proximal colon cancer by about 70 % (incidence–0.35, 0.20–0.63; mortality–0.24, 0.11–0.52, both p < 0.0001) and also reduced risk of rectal cancer (incidence–0.58, 0.36–0.92, p = 0.02; mortality–0.47, 0.26–0.87, p = 0.01). Results were consistent across trials, with no increase in benefit at doses of aspirin above 75 mg daily, and an absolute reduction in 20-year risk of any fatal colorectal cancer after 5 year’s scheduled treatment with 75–300 mg daily of 1.76 % (0.61–2.91, p = 0.001). However, there was a trend towards a higher risk of fatal colorectal cancer on 30 versus 283 mg daily on long-term follow-up of the Dutch TIA trial (OR = 2.02, 0.70–6.05, p = 0.15) (Rothwell et al. 2010; Singh et al. 2010; Brenner et al. 2010).
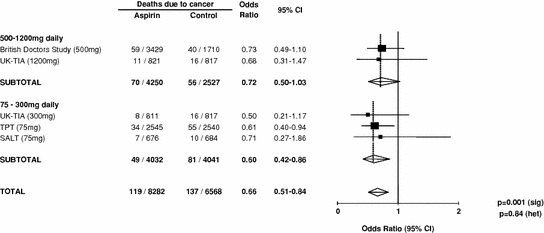
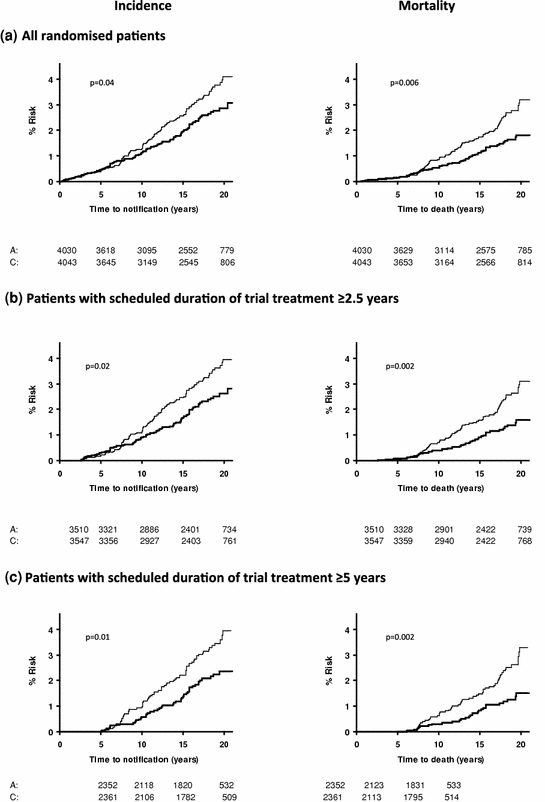
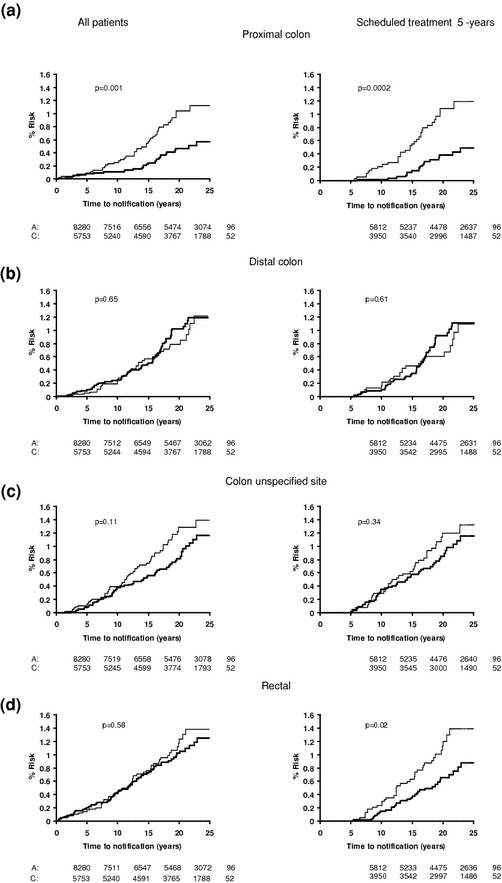

Fig. 2
Meta-analysis of effect of aspirin on long-term risk of death due to colorectal cancer in randomised trials of aspirin versus control (Data derived form Rothwell et al. 2010). Note to subeditor: the TOTAL line should read as follows in order to avoid double counting of the UK-TIA placebo group: 119/8,282 vs 121/5,751 OR = 0.66, 95 % CI 0.51 − 0.85, p = 0.002 (sig); p = 0.84 (het)

Fig. 3
Pooled analysis of the effect of aspirin (thick line) versus control (thin line) on subsequent incidence and mortality due to colorectal cancer in all randomised patients (a) in three trials of low-dose (75–300 mg daily) aspirin versus placebo, in those with scheduled duration of trial treatment ≥2.5 years (b), and in those with scheduled duration of trial treatment ≥5 years (c) Rothwell et al. 2010). a All randomised patients. b Patients with scheduled duration of trial treatment ≥2.5 years

Fig. 4
Pooled analysis of the effect of aspirin (75–1,200 mg; thick line) versus control (thin line) on incidence of colorectal cancer by site during and after four randomised patients (Rothwell et al. 2010). a Proximal colon. b Distal colon. c Colon unspecified site. d Rectal
The finding of greater effects of aspirin on cancers of the proximal colon versus distal colon and rectum was unexpected and may simply be due to chance. Most observational studies have found no consistent differences in associations among aspirin use and risks of colon cancer versus rectal cancer, and reports of differences in effect by site of colon cancer have been inconsistent (Flossmann and Rothwell 2007). However, the only randomised trial of aspirin in prevention of recurrent adenomas to report results by site did report a 40–50 % reduction in proximal colonic adenomas with aspirin and no reduction in distal adenomas (Wallace et al. 2009), and in the analysis of randomised trials of aspirin by Rothwell et al. (2010), the difference in effect of aspirin between proximal and distal colon cancers was statistically significant for both incidence and mortality, was present at all doses of aspirin and consistent in all four trials of aspirin versus control. Moreover, differences in effects of other treatments on proximal versus distal colon tumours are widely accepted (Elsaleh et al. 2000), and there are many differences in normal physiology between the proximal and distal colon (Bufill 1990; Iacopetta 2002), due partly to their different embryological origins and in risk factors for cancers in the two sites, in mechanisms of carcinogenesis, and in the molecular and genetic characteristics of the cancers (Bufill 1990; Iacopetta 2002; Birkenkamp-Demtroder et al. 2005; Yamauchi et al. 2012; Leopoldo et al. 2008), Of potential relevance, expression of COX-2 tends to be greater in tumours of the distal colon and rectum than in tumours of the proximal colon (Birkenkamp-Demtroder et al. 2005; Chapple et al. 2000; Nasir et al. 2004), aspirin, therefore, perhaps achieves less complete inhibition of COX-2 in distal tumours. Follow-up of other aspirin trial cohorts should provide more evidence of the relative effects on proximal versus distal cancers. Irrespective of this, the proven prevention of proximal colonic cancers by aspirin, which would not be identified by sigmoidoscopy and which are often missed on colonoscopy, (Singh et al. 2010; Brenner et al. 2010)is clearly important, and suggests that these two approaches to prevention of colorectal cancer may be synergistic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







