ESSENTIAL CONCEPTS
ESSENTIAL CONCEPTS
Gut-associated lymphoid tissues (GALT) are characterized by a unique structure, physiologic inflammation, a tendency to suppress immune responses (oral tolerance), and production of secretory immunoglobulins.
The immune response has two major arms: innate (rapid, hard-wired) and adaptive (delayed in onset with memory).
Inflammatory bowel disease (IBD) offers a paradigm for understanding and treating intestinal inflammatory diseases.
IBD is a dysregulated immune response of GALT to normal commensal microbes within the intestine; risk of IBD is altered by genetic susceptibility and by specific environmental factors (eg, tobacco).
Numerous genetic loci and environmental elements defined as risk factors for IBD regulate innate and adaptive immunity, the epithelial barrier, and the relationships of each of these with normal commensal microbiota (bacterial and nonbacterial).
IBD is ultimately caused by overproduction of proinflammatory mediators relative to anti-inflammatory mediators, both of which are derived from cells associated with adaptive immunity (T helper [Th] cells) and innate immunity (macrophages and dendritic cells) that excessively infiltrate the intestinal tissues.
Although Crohns disease (CD) may preferentially exhibit overactivity of Th1 and Th17 cells, and ulcerative colitis (UC) may exhibit overactivity of Th2 cells, there is significant overlap in the immunopathogenesis of these disorders.
Excess production of cytokines derived from innate immune pathways (tumor necrosis factor [TNF] and interleukin-6 [IL-6]) occurs in both CD and UC.
T regulatory cells secrete anti-inflammatory cytokines (eg, IL-10, transforming growth factor-β [TGFβ], and IL-35) that inhibit proinflammatory cytokine responses from innate and adaptive immune cells.
Increased understanding of IBD immunopathogenesis has led to development of therapeutic agents that are increasingly being administered in a logical, mechanism-based manner.
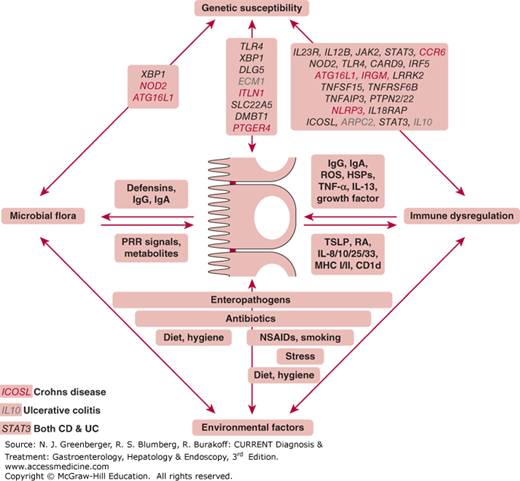
Clinically, IBD is a chronic inflammatory condition of the intestines that is marked by remission and relapses and distills clinically into one of two major subtypes of disease: UC and CD. Both diseases have a general commonality in their pathogenesis and are derived from a dysregulated mucosal immune response to antigenic components of the normal commensal microbiota that reside within the intestine (Figure 2–1).
Figure 2–1.
Pathophysiologic mechanisms of inflammatory bowel disease (IBD). IBD represents the dysregulated mucosal immune response to commensal microbial antigens that is modified by genetic and environmental risk factors. At its core, IBD derives from dysfunctional bidirectional interactions between the commensal microbiota, the intestinal epithelial cells, and the immune system components within the epithelium and lamina propria. Numerous genetic and environmental factors have been identified that modify the risk for IBD development by specifically affecting the composition and function of the aforementioned critical core elements of disease pathogenesis (commensal microbiota, intestinal epithelium, or immune system). Examples of factors that are involved in these pathways are shown. HSP, heat shock proteins; NSAID, nonsteroidal anti-inflammatory drugs; PRR, pattern recognition receptors; RA, retinoic acid; ROS, reactive oxygen species; TSLP, thymic stromal lymphopoietin; Genes’ names in red are Crohn disease specific, blue are ulcerative colitis specific, and black are those that affect both diseases. (Adapted, with permission, from Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621.)
At its core, IBD (both UC and CD) represents a disturbed relationship between the commensal microbiota, intestinal epithelial cells (IECs), and the immune system associated with intestinal tissues. This tripartite interaction between the mucosal immune response, epithelial barrier, and commensal microbiota is increasingly recognized to be specifically influenced by poorly understood environmental factors and genetic elements that affect the lifetime risk of developing this disorder. Although the influence of specific gene variations, as endogenous risk factors that clearly define susceptibility to this disease, is well accepted, only a limited number of environmental factors have clearly been proven to either modify these diseases or regulate the lifetime risk of developing them. These include tobacco use, enteropathogenic exposures, appendectomy, antibiotic use, and oral contraceptive pills. These do not, in and of themselves, cause the disease but likely modify the genetically defined or undefined aspects of the most critical components that underlie the immunopathogenesis of this disease: intestinal bacteria, epithelial barrier, and mucosal immune response. Of the latter three factors, the best understood influence—which has, to date, generated the most information, resulting in novel and exciting new forms of therapy—involves the mucosal immune response associated with these disorders. For this reason, a discussion of the immunopathogenesis of IBD not only is important for understanding the pathophysiology of these diseases but also provides a basis for understanding both the mechanisms and the rationale for using the wide variety of therapeutic approaches that have recently been developed or are soon to be developed for the treatment of these diseases.
[PubMed: 20192811]
[PubMed: 15168363]
[PubMed: 11861611]
[PubMed: 17345609]
GENERAL PRINCIPLES OF THE GUT-ASSOCIATED LYMPHOID TISSUES
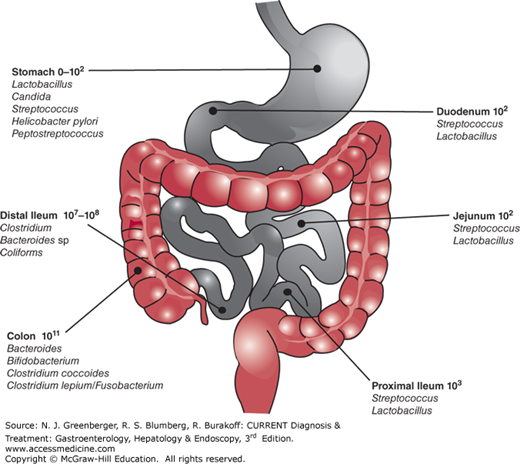
The normal intestine has an enormous surface area that is exposed to a wide variety of exogenous, especially bacterial, antigens (Figure 2–2). Consequently, the mucosal immune system has had to develop a wide variety of very specific modifications and developmental structures to deal with and respond to these challenges. Importantly, the overwhelming majority of initial antigen encounters for the host occur at the mucosal surface, which is bathed by a heterogeneous population of microorganisms, most of which are congregated within the colon and distal small intestine where IBD most commonly occurs. Thus, the intestines are confronted by a large number of antigenic stimuli that must be deciphered for pathologic potential.
For the majority of antigenic challenges, a response that is characterized by either ignorance or active suppression would seem to be the most appropriate in the intestine to avoid a nonspecific inflammatory response. Obviously, for a few exposures such as to pathogens, a robust immune response is appropriate. The mucosal-associated lymphoid tissues associated with the gut, or so called GALT, are characterized by a unique organization and regulated state of controlled (or physiologic) inflammation. Thus, the gut is poised for, but actively restrained from, full action and notable for a tendency to suppress responses, a characteristic referred to as oral tolerance. Oral tolerance and controlled inflammation are unique hallmarks of the GALT and are both manifestations of the tendency of mucosal-associated lymphoid tissues to tightly regulate immune responses (Table 2–1).
These two functional hallmarks of the GALT are representative of the fact that the intestine is a very large immunologic organ in addition to its obvious other functions associated with digestion and endocrine secretion. Its functions are organized anatomically and include two components. The first component is the so-called organized GALT, which includes the Peyer patches, isolated lymphoid follicles, and lymphocyte-filled villi. The second component, the so-called diffuse GALT, comprises anatomic structures that are diffusely contained within the lamina propria.
The organized GALT structures are often characterized by specialized types of epithelium such as the so-called microfold cell (M cell). M cells are unique epithelial cells that overly the Peyer patches, with its rich content of associated lymphocytes and dendritic cells that allow for selective uptake of and response to distinct types of antigens. The Peyer patches and other organized lymphoid structures are distributed throughout the gastrointestinal tract but are especially congregated in the distal ileum. They are mainly inductive sites where antigens, including bacterial antigens, are taken up, processed, and presented by dendritic cells for the education of the GALT-associated lymphocytes.
The diffuse GALT constitutes the majority of lymphoid tissue within the small and large intestine and is characterized by a single layer of simple, columnar epithelial cells that separates the lumen of the intestines from the lamina propria. This epithelium and its associated intraepithelial lymphocytes, and underlying scattered lymphoid and dendritic cells are a major effector site for protecting the large surface of intestines that must be defended from epithelial exposures to pathogens. In addition, this epithelial surface is highly responsible for participating in the maintenance of a regulated immune response to the wide variety of microbes associated with the normal commensal microbiota.
Both the epithelial compartment above the epithelial basement membrane and the subepithelial compartment below the epithelial basement membrane that is contained within the lamina propria participate in the two major arms of the immune system. These are the innate and adaptive (or specific) immune systems (Table 2–2).
| Type of Immunity | Receptor | Ligand | Cell Type |
|---|---|---|---|
| Innate immunity (rapid, hard wired) | TLR2 | Peptidoglycan | Macrophages |
| TLR4 | LPS | Macrophages/IEC | |
| TLR5 | Flagellin | Macrophages/IEC | |
| TLR9 | Bacterial DNA | Dendritic cells | |
| CRP | Bacterial carbohydrate | Serum | |
| NOD2 | Muramyl dipeptide | Dendritic cells/IEC | |
| Adaptive or specific immunity (delayed with memory) | TCR | HLA plus peptide | T cell |
| BCR | Immunoglobulin | B cell | |
| CD28 | CD80, CD86, CTLA44 | T cell |
The innate immune system contains a pattern recognition system that provides a hard-wired and rapid response system for responding to microbial structures. A classic group of structures that are used by a wide variety of cell types in an innate immune response is the so-called toll-like receptors (TLRs), which respond to microbial structures as diverse as lipopolysaccharide or DNA of microbes. Another major class of pattern recognition receptors associated with innate immunity is the nucleotide oligomerization domain (NOD)-like receptors (NLRs), which recognize microbial structures such as muramyl dipeptide from the peptidoglycans of gram-negative and gram-positive bacteria. Innate, pattern-recognition receptors are distributed on virtually all cell types but are especially congregated on professional (dendritic cells, macrophages, and B cells) and nonprofessional (intestinal epithelial cells) antigen-presenting cells (APCs).
Adaptive or specific immunity has a delayed response and is characterized by memory. This type of immunity is characteristic of the immune response derived from T and B cells and requires the uptake, processing, and presentation of antigens by APCs to the lymphoid cell types (T and B cells) associated with the adaptive immune response. Moreover, innate and adaptive immunity interact with each other such that they both promote and regulate each other in the generation of a balanced and effective immune response.
[PubMed: 19079158]
[PubMed: 17680011]
[PubMed: 21150896]
PATHOGENESIS OF INFLAMMATORY BOWEL DISEASE
The major operating paradigm of our current understanding of IBD is that this disease represents hyperreactivity or loss of (oral) tolerance of the mucosal immune system to one’s own mucosal microbiota. This is consistent with the general localization of the disease, both UC and CD, to the regions of the intestine where microbes are mostly present. This concept is mainly derived from observations with numerous animal models of IBD, as well as a lesser number of human clinical studies. The latter have revealed some response to antibiotics, especially in CD, and the lack of any identifiable pathogens in both clinical subtypes of IBD. Thus, IBD likely represents a dysregulated mucosal immune response in a genetically susceptible host to commensal microbial antigens. At the same time, the commensal microbiota provides numerous protective signals that assist in the maintenance of homeostasis and the prevention of inflammation. Thus, IBD is associated with an increase in microbiota that tend to promote inflammation (eg, proteobacteria such as enteroadherent and invasive Escherichia coli) relative to the quantity of microbes that are inclined to prevent inflammation (eg, Firmicutes such as Faecalibacterium prausnitzii).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree