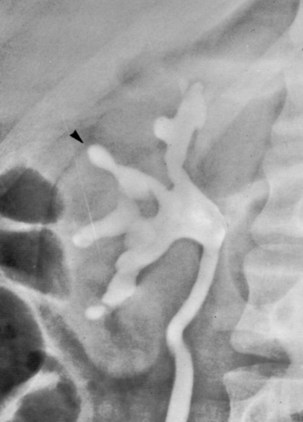
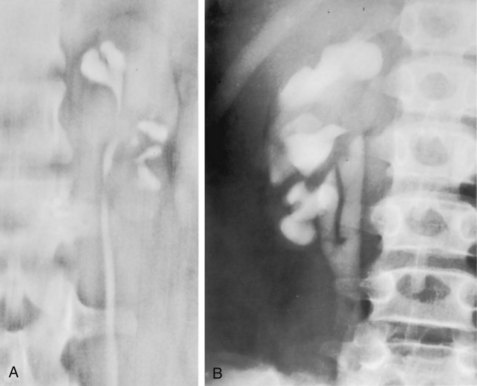
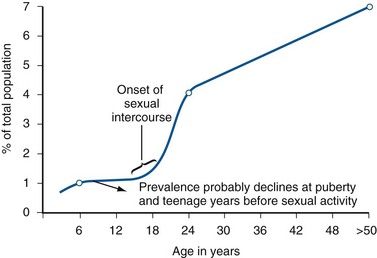
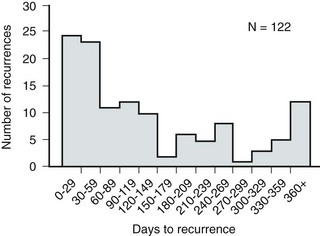
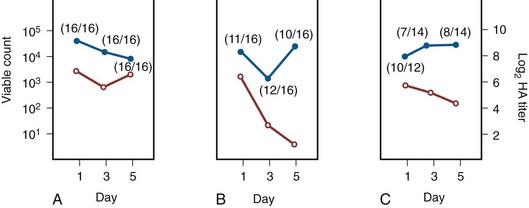
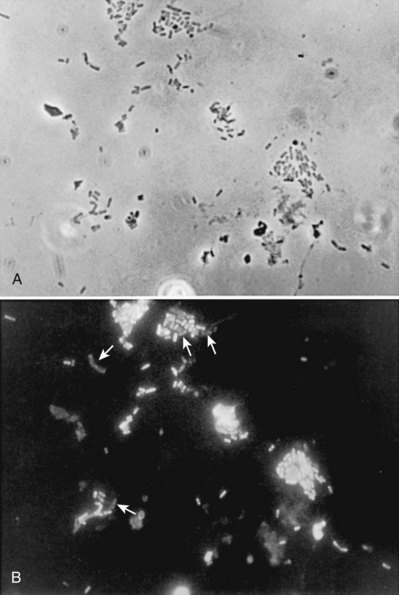
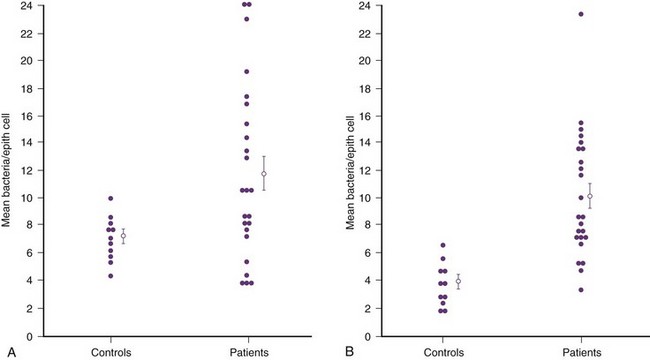
Anthony J. Schaeffer, MD, Edward M. Schaeffer, MD, PhD Bacteriuria is the presence of bacteria in the urine, which is normally free of bacteria. It has been assumed to be a valid indicator of either bacterial colonization or infection of the urinary tract. Although this is usually true, studies in animals (Hultgren et al, 1985; Mulvey et al, 1998) and humans (Elliott et al, 1985) have indicated that bacteria may be in the urothelium in the absence of bacteriuria. Alternatively, bacteriuria may represent bacterial contamination of an abacteriuric specimen during collection. Infections are often defined clinically by their presumed site of origin. Chronic pyelonephritis describes a shrunken, scarred kidney, diagnosed by morphologic, radiologic, or functional evidence of renal disease that may be postinfectious but is frequently not associated with UTI. Bacterial infection of the kidney may cause a focal, coarse scar in the renal cortex overlying a calyx, almost always accompanied by some calyceal distortion (Fig. 10–1), which can be detected radiographically or by gross examination of the kidney. Less commonly, renal scarring from infection can result in atrophic pyelonephritis or generalized thinning of the renal cortex, with a small kidney appearing radiographically similar to one with postobstructive atrophy (Fig. 10–2). Figure 10–2 A, Excretory urogram of the contralateral left kidney from the same patient as in Figure 10–1. The severe pyelonephritic atrophy, undoubtedly caused by febrile urinary infections during early infancy with reflux into different segments of the kidney, produced irregular cortical scarring. Note how all the calyces extend to the capsule with irregular, intervening areas of cortex. B, Pyelonephritic atrophy, suggestive of postobstructive atrophy, in a 20-year-old woman with spina bifida, neurogenic bladder, and many episodes of fever and bacteriuria in early childhood. Observe the uniform, regular atrophy of the renal cortex that suggests reflux of bacteria simultaneously into virtually all nephrons. This type of pyelonephritic atrophy is uncommon compared with that shown in A and is characteristic of obstruction with superimposed infection. A complicated infection is associated with factors that increase the chance of acquiring bacteria and decrease the efficacy of therapy (Table 10–1). The urinary tract is structurally or functionally abnormal, the host is compromised, and/or the bacteria have increased virulence or antimicrobial resistance. The majority of these patients are men. Table 10–1 Factors That Suggest Complicated UTI Reprinted from Schaeffer AJ. Urinary tract infections. In: Gillenwater JY et al, editors. Adult and pediatric urology. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 212. UTIs may also be defined by their relationship to other UTIs: UTIs are considered to be the most common bacterial infection. They account for more than 7 million visits to physicians’ offices and necessitate or complicate over 1 million office visits and 1 million emergency department visits, resulting in 100,000 hospitalizations annually (Patton et al, 1991, Hooton and Stamm, 1997; Foxman et al, 2000). They account for 1.2% of all office visits by women and 0.6% of all office visits by men (Schappert, 1997). The overall prevalence of bacteriuria in women has been estimated at 3.5%, with prevalence generally increasing with age in a linear trend (Evans et al, 1978). Surveys screening for bacteriuria have shown that about 1% of schoolgirls (aged 5 to 14 years) (Kunin et al, 1962) have bacteriuria and that this figure increases to about 4% by young adulthood and then by an additional 1% to 2% per decade of age (Fig. 10–3). Nearly 30% of women will have had a symptomatic UTI requiring antimicrobial therapy by age 24, and almost half of all women will experience a UTI during their lifetime. The prevalence of bacteriuria in young women is 30 times more than in men. However, with increasing age, the ratio of women to men with bacteriuria progressively decreases. At least 20% of women and 10% of men older than 65 years have bacteriuria (Boscia and Kaye, 1987; Juthani-Mehta, 2007). Figure 10–3 Prevalence of bacteriuria in females as a function of age. (From Stamey TA. The prevention of recurrent urinary infections. New York: Science and Medicine; 1973.) The incidence of bacteriuria also increases with institutionalization or hospitalization and concurrent disease (Sourander, 1966). In a study of women and men older than 68 years, Boscia and Kaye (1987) found that 24% of functionally impaired nursing home residents had bacteriuria compared with 12% of healthy domiciliary subjects (Boscia et al, 1986). UTIs account for approximately 38% of the 2 million nosocomial infections each year (Sedor and Mulholland, 1999; Lo et al, 2008). More than 80% of nosocomial UTIs are secondary to an indwelling urethral catheter (Sedor and Mulholland, 1999). The incidence of UTIs is also increased during pregnancy and in patients with spinal cord injuries, diabetes, multiple sclerosis, and human immunodeficiency virus (HIV) infection/acquired immunodeficiency syndrome (AIDS). The financial impact of community-acquired UTIs is nearly $1.6 billion in the United States alone (Foxman, 2002); the annual cost of nosocomial UTIs has been estimated to range from between $515 million and $548 million (Jarvis, 1996). Little is known about the natural history of untreated bacteriuria in women because most women are treated when they are diagnosed, but a few studies in which treatment with antimicrobial agents is compared with placebo have been done. These show that 57% to 80% of bacteriuric women who are untreated or treated with placebo clear their infections spontaneously (Mabeck, 1972; Guttmann, 1973). Mabeck (1972) found that 8 of 53 bacteriuric women placed on placebo needed treatment with an antimicrobial agent because of symptoms, but 32 of the remaining 45 women cleared without treatment within a month, and 43 of the 45 had spontaneously cleared of bacteriuria within 5 months; only 2 women remained persistently bacteriuric. Once a patient has an infection, he or she is likely to develop subsequent infections. Many adults had UTIs as children, underscoring the importance of genotypic factors in UTIs (Gillenwater et al, 1979). Of 45 women with untreated UTIs whose infection cleared, 20 (46%) had recurrences within a year (Mabeck, 1972). When women with recurrent bacteriuria were observed after treatment, about one sixth (37 of 219) had a very high recurrence rate (2.6 infections per year) whereas the remaining women had a recurrence rate of only 0.32 per year (Mabeck, 1972). Similar separation was seen in a prospective study in which only 28.6% of 60 women who experienced their first symptomatic UTI had recurrent infections over the first 18 months of observation, as opposed to recurrences in 82.5% of 106 women who had had previous UTIs (Harrison et al, 1974). Other investigators also have found that the probability of recurrent UTIs increases with the number of previous infections and decreases in inverse proportion to the elapsed time between the first and the second infections (Mabeck, 1972). Of these recurrent infections, 71% to 73% are caused by reinfection with different organisms, rather than recurrence with the same organism (Mabeck, 1972; Guttmann, 1973). Women with frequent reinfections have a rate of 0.13 to 0.25 UTIs per month (1.6 to 3.1 infections per year) when the infections are treated with antimicrobial agents (Mabeck, 1972; Guttmann, 1973; Kraft and Stamey, 1977; Vosti, 2002). In a prospective long-term study of 235 women with more than 1000 confirmed infections studied over a period ranging from 1 to nearly 20 years, about half of the patients had clusters of infections, which ranged in frequency from 2 to 12 infections per cluster. Infections were followed by remission-free intervals that averaged approximately 1 year. Most reinfections occurred after 2 weeks (Harrison et al, 1974) and within 5 months (Mabeck, 1972), and most occurred early in this interval (Kraft and Stamey, 1977; Vosti, 2002) (Fig. 10–4). Rates of reinfection were independent of bladder dysfunction, radiologic changes of chronic pyelonephritis, and vesicoureteral reflux (Guttmann, 1973). The reinfections did not occur evenly over time. In the Stanford series (Kraft and Stamey, 1977), 23 women with frequent recurrent infections were studied with monthly urine cultures when asymptomatic and with immediate cultures when symptomatic for cystitis, for a mean of 3 years. Thirty-four percent of infections were followed by infection-free intervals of at least 6 months (average, 12.8 months), and 22 of the 23 women had such intervals. However, even these long intervals were followed by further infections (Kraft and Stamey, 1977), thus underscoring the importance of genotypic factors in the pathogenesis of UTIs in women (Schaeffer et al, 1981). Figure 10–4 Days between recurrent UTIs grouped by 30-day intervals. (From Kraft JK, Stamey TA. The natural history of symptomatic recurrent bacteriuria in women. Medicine 1977;56:55.) When the Stanford data (Kraft and Stamey, 1977) on recurrent UTIs in highly susceptible females are analyzed by examining sets of infections separated by remissions of at least 6 months, 69% of the sets contain only one infection. After this first set, the remaining sets show a 33% remission rate in infections, which means a patient who has two or more infections within 6 months has only a 33% probability of remaining free of infection for the next 6 months. Therefore, if antimicrobial prophylaxis is started after the second or any succeeding infection within a set, about two thirds of the women will benefit. Whether a patient receives no treatment at all or short-term, long-term, or prophylactic antimicrobial treatment the risk of recurrent bacteriuria remains the same; prophylactic antimicrobial therapy reduces reinfections but does not alter the underlying predisposition to recurring infection. Asscher and associates (1973) found that reinfections occurred in 17 patients (34%) treated with a 7-day course of nitrofurantoin and in 13 patients (29%) receiving placebo during a 3- to 5-year follow-up. Mabeck (1972) found that 46% (20 of 43) of untreated patients had recurrent infections by 12 months compared with about 40% of treated patients who had recurrences. Both studies suggest that it makes little difference whether a UTI is cured with an antimicrobial agent or is allowed to clear spontaneously—the susceptibility to recurrent UTI remains the same. Moreover, patients with frequent UTI who take prophylactic antimicrobial agents for extended periods (≥6 months) may decrease their infections during the time of prophylaxis but the rate of infection returns to the pretreatment rate after prophylaxis is stopped (Vosti, 1975; Stamm et al, 1980a). Even long interruptions in the pattern of recurrence, therefore, do not appear to alter the patient’s basic susceptibility to infections. The sequelae of complicated UTIs are substantial. It is well established in the presence of obstruction, infection stones, diabetes mellitus, and other risk factors that UTIs in adults can lead to progressive renal damage (Freedman, 1975). The long-term effects of uncomplicated recurrent UTIs are not completely known, but, so far, no association between recurrent infections and renal scarring, hypertension, or progressive renal azotemia has been established (Asscher et al, 1973; Freedman, 1975). Indeed, one investigator was unable to find a single case of unequivocal nonobstructive chronic pyelonephritis in 22 patients in whom chronic pyelonephritis was the cause of end-stage renal failure (Schechter et al, 1971). Similar data were reported by Huland and Busch (1982). Key Points Incidence and Epidemiology Most bacteria enter the urinary tract from the bowel reservoir via ascent through the urethra into the bladder. Adherence of pathogens to the introital and urothelial mucosa plays a significant role in ascending infections. This route is further enhanced in individuals with significant soilage of the perineum with feces, women who use spermicidal agents (Hooton et al, 1996; Foxman, 2002; Handley et al, 2002), and patients with intermittent or indwelling catheters. Although cystitis is often restricted to the bladder, approximately 50% of infections can extend into the upper urinary tract (Busch and Huland, 1984). The weight of clinical and experimental evidence strongly suggests that most episodes of pyelonephritis are caused by retrograde ascent of bacteria from the bladder through the ureter to the renal pelvis and parenchyma. Although reflux of urine is probably not required for ascending infections, edema associated with cystitis may cause sufficient changes in the ureterovesical junction to permit reflux. Once the bacteria are introduced into the ureter, they may ascend to the kidney unaided. However, this ascent would be greatly increased by any process that interferes with the normal ureteral peristaltic function. Gram-negative bacteria and their endotoxins, as well as pregnancy and ureteral obstruction, have a significant antiperistaltic effect. Infection of the kidney by the hematogenous route is uncommon in normal individuals. However, the kidney is occasionally secondarily infected in patients with Staphylococcus aureus bacteremia originating from oral sites or with Candida fungemia. Experimental data indicate that infection is enhanced when the kidney is obstructed (Smellie et al, 1975). E. coli is by far the most common cause of UTIs, accounting for 85% of community-acquired and 50% of hospital-acquired infections. Other gram-negative Enterobacteriaceae, including Proteus and Klebsiella, and gram-positive E. faecalis and Staphylococcus saprophyticus are responsible for the remainder of most community-acquired infections. Nosocomial infections are caused by E. coli, Klebsiella, Enterobacter, Citrobacter, Serratia, Pseudomonas aeruginosa, Providencia, E. faecalis, and S. epidermidis (Kennedy et al, 1965). Less common organisms such as Gardnerella vaginalis, Mycoplasma species, and Ureaplasma urealyticum may infect patients with intermittent or indwelling catheters (Josephson et al, 1988; Fairley and Birch, 1989). The prevalence of infecting organisms is influenced by the patient’s age. For example, S. saprophyticus is now recognized as causing approximately 10% of symptomatic lower UTIs in young, sexually active females (Latham et al, 1983) whereas it rarely causes infection in males and elderly individuals. A seasonal variation with a late summer to fall peak has been reported (Hovelius and Mardh, 1984). Although symptomatic anaerobic infections of the urinary tract are documented, they are uncommon. However, the distal urethra, perineum, and vagina are normally colonized by anaerobes. Whereas 1% to 10% of voided urine specimens are positive for anaerobic organisms (Finegold, 1977), anaerobic organisms found in suprapubic aspirates are much more unusual (Gorbach and Bartlett, 1974). Clinically symptomatic UTIs in which only anaerobic organisms are cultured are rare, but these organisms must be suspected when a patient with bladder irritative symptoms has cocci or gram-negative rods seen on microscopic examination of the centrifuged urine (catheterized, suprapubic aspirated, or voided midstream urine) and routine quantitative aerobic cultures fail to grow organisms (Ribot et al, 1981). Anaerobic organisms are frequently found in suppurative infections of the genitourinary tract. In one study of suppurative genitourinary infections in males, 88% of scrotal, prostatic, and perinephric abscesses included anaerobes among the infecting organisms (Bartlett and Gorbach, 1981). The organisms found are usually Bacteroides species, including B. fragilis, Fusobacterium species, anaerobic cocci, and Clostridium perfringens (Finegold, 1977). The growth of clostridia may be associated with cystitis emphysematosa (Bromberg et al, 1982). Mycobacterium tuberculosis and other nontuberculous mycobacteria may be found when cultures for acid-fast bacteria are requested; they do not grow under routine aerobic conditions and may be found during evaluation for sterile pyuria. It has been emphasized that the mere presence of mycobacteria may not indicate tissue invasion. Therefore factors such as symptoms, endoscopic or radiologic evidence of infection, abnormal urine sediment, the absence of other pathogens, repeated demonstration of the organism, and the presence of granulomas should be considered before therapy is instituted (Brooker and Aufderheide, 1980; Thomas et al, 1980). (M. tuberculosis is discussed in Chapter 16.) Virulence characteristics play a role in determining both if an organism will invade the urinary tract and the subsequent level of infection within the urinary tract. It is generally believed that uropathogenic strains resident in the bowel flora, such as uropathogenic E. coli (UPEC), can infect the urinary tract not only by chance but also by the expression of virulence factors that enable them to adhere to and colonize the perineum and urethra and migrate to the urinary tract where they establish an inflammatory response in the urothelium (Schaeffer et al, 1981; Yamamoto et al, 1997; Schlager et al, 2002; Moreno et al, 2008). The same virulence factors can be found on bacterial strains that cause recurrent UTI in patients (Foxman et al, 1995). Some of these virulence determinants are located on one of approximately 20 UPEC-specific pathogenicity-associated islands ranging from 30 to 170 kb (Hacker, 1999; Oelschlaeger et al, 2002). These pathogenicity islands collectively increase the size of the pathogen genome by about 20% over a commensal strain. A recent genomic analysis of a UPEC strain revealed the presence of genes for putative chaperone-usher systems as well as autotransporter proteins that may function as adhesins, toxins, proteases, invasins, serum resistance factors, or motility mediators (Henderson and Nataro, 2001). One UPEC-specific autotransporter, Sat, seems toxic to urinary tract cells in vitro (Guyer et al, 2000) and can cause cytoplasmic vacuolation and severe histologic damage in mouse kidneys (Guyer et al, 2002). Another toxin, hemolysin HlyA, forms pores in a variety of host cell membranes (Uhlen et al, 2000). In addition to proteases and toxins, UPEC produces several iron acquisition systems, including aerobactin (Johnson et al, 1988; Johnson, 2003) and the more recently described IroN system (Russo et al, 1999; Sorsa et al, 2003). Lastly, most UPEC strains produce an acid polysaccharide capsule that protects the bacteria from phagocytosis by human polymorphonuclear leukocytes and inhibits activation of complement (Johnson, 2003). Bacterial adherence to vaginal and urothelial epithelial cells is an essential step in the initiation of UTIs. This interaction is influenced by the adhesive characteristics of the bacteria, the receptive characteristics of the epithelial surface, and the fluid bathing both surfaces. Bacterial adherence is a specific interaction that plays a role in determining the organism, the host, and the site of infection. Portions of this section on bacterial adherence have been published (Schaeffer et al, 1981). UPEC expresses a number of adhesins that allow it to attach to urinary tract tissues (Mulvey, 2002). These adhesins are classified as either fimbrial or afimbrial, depending on whether the adhesin is displayed as part of a rigid fimbria or pilus (Fig. 10–5). Bacteria may produce a number of antigenically and functionally different pili on the same cell; others produce a single type; in some, no pili are seen (Klemm, 1985). A typical piliated cell may contain 100 to 400 pili. The pilus is usually 5 to 10 nm in diameter, is up to 2 µm long, and appears to be composed primarily of subunits known as pilin (Klemm, 1985). Pili are defined functionally by their ability to mediate hemagglutination of specific types of erythrocytes. The most well-described pili are types 1, P, and S. Type 1 pili are commonly expressed on both nonpathogenic and pathogenic E. coli. Type 1 pili consist of a helical rod composed of repeating FimA subunits joined to a 3-nm wide distal tip structure containing the adhesin FimH (Jones et al, 1995). These pili mediate hemagglutination of guinea pig erythrocytes (Duguid et al, 1979). The reaction is inhibited by the addition of mannose; thus type 1 pili are termed mannose-sensitive hemagglutination (MSHA) (Svenson et al, 1984; Reid and Sobel, 1987). The role of type 1 pili as a virulence factor in UTIs has been established. This evidence has been obtained (1) from the analysis of bacteria isolated from the urine of patients with UTIs, which were found to express mannose-sensitive (MS) adhesins (Ljungh and Wadstrom, 1983); (2) from studies with animal models (Fader and Davis, 1982; Hagberg et al, 1983a, 1983b; Iwahi et al, 1983; Hultgren et al, 1985) in which inoculation of type 1 piliated organisms into the bladder resulted in significantly more colonization of the urinary tract than inoculation of nonpiliated organisms; and (3) from the observation that anti-type 1 pili antibodies and competitive inhibitors such as methyl-α-D-mannopyranoside protected mice from contracting UTIs (Aronson et al, 1979; Hultgren et al, 1985). Recent studies have demonstrated that interactions between FimH and receptors expressed on the luminal surface of the bladder epithelium are critical for the ability of many UPEC strains to colonize the bladder and cause disease (Connell et al, 1996; Langermann et al, 1997; Thankavel et al, 1997; Mulvey et al, 1998). P (Mannose Resistant) Pili. P pili confer tropism to the kidney, the designation “P” standing for pyelonephritis (Mulvey, 2002). P pili, which are found in most pyelonephritogenic strains of UPEC, mediate hemagglutination of human erythrocytes that is not altered by mannose and is thus termed mannose-resistant hemagglutination (MRHA) (Kallenius et al, 1979). The adhesin PapG, at the tip of the pilus, recognizes the α-D-galactopyranosyl-(1-4)-β-D-galactopyranoside moiety present in the globoseries of glycolipids (Kallenius et al, 1980; Leffler and Svanborg-Eden, 1980), which are found on P-blood group antigens and on uroepithelium (Svenson et al, 1983). The MRHA adhesins of UPEC that do not show the digalactoside-binding specificity have been provisionally named X adhesins (Vaisanen et al, 1981). In some strains of UPEC, hemagglutination is mediated by nonpiliated adhesins or hemagglutinins (Duguid et al, 1979). Svanborg-Eden and coworkers (1978) were the first to report a correlation between bacterial adherence and severity of UTIs. They showed that UPEC strains from girls with acute pyelonephritis had high adhesive ability whereas strains causing asymptomatic bacteriuria or from the feces of healthy girls had low bacterial adherence. Between 70% and 80% of the pyelonephritic strains, but only 10% of the bowel isolates, had adhesive capacity. Furthermore, P pili were present in 91% of urinary strains causing pyelonephritis, 19% of strains causing cystitis, and 14% of strains causing asymptomatic bacteriuria but only 7% of bowel isolates from healthy children, highlighting the correlation between bacterial adherence and UTIs (Kallenius et al, 1981). Whereas MRHA and P pili are strongly associated with pyelonephritis, these virulence factors are not associated with renal scarring and reflux due to bacterial infection (Vaisanen et al, 1981). Studies suggest minimal correlation between P-piliated E. coli strains and recurrent pyelonephritis with gross reflux in girls (Lomberg et al, 1983). Thus it would appear that P pili in acute pyelonephritis are important mainly in nonrefluxing or minimally refluxing children. S pili, which bind to sialic acid residues via the SfaS adhesin, have been associated with both bladder and kidney infection (Mulvey, 2002). F1C pili bind to glycosphingolipids in renal epithelial cells and induce an interleukin-8 inflammatory response (Backhed et al, 2002). UPEC also expresses a group of afimbrial adhesins (AFA), which have been clustered with the Dr adhesin family for their recognition of decay-accelerating factor and for their similar genetic structure. Decay-accelerating factor is found on numerous different epithelial sites, and Dr adhesins are known to bind to many locations throughout the urinary tract (Anderson et al, 2004b). Early evidence for the role of type 1 and P pili in adherence in UTIs in humans was contradictory. Pili were visible by electron microscopy on E. coli in the urine of 31 of 37 patients (Ljungh and Wadstrom, 1983). Conversely, no MS adhesins were found in 22 of 24 urine isolates from patients with indwelling catheters (Ofek et al, 1981), and 19 of 20 samples from patients with acute UTIs were devoid of pili and nonadherent until subcultured in broth (Harber et al, 1982). Assessment of pili production by clinical E. coli isolates demonstrates that environmental growth conditions can produce rapid changes in pilus expression (Duguid et al, 1966; Goransson and Uhlin, 1984; Hultgren et al, 1986), wherein cells switch back and forth between piliated and nonpiliated phases (Eisenstein, 1981). For example, some bacteria grown in a broth medium express pili whereas the same strain grown on the same medium in a solid state will cease production of pili. This process, called phase variation, can also occur in vivo and has obvious biologic and clinical implications. For example, the presence of type 1 pili may be advantageous to the bacteria for adhering to and colonizing the bladder mucosa but disadvantageous because the pili enhance phagocytosis and killing by neutrophils (Silverblatt et al, 1979). When bladder and kidney cultures were examined 1, 3, and 5 days after intravesical inoculation of piliated bacteria, organisms recovered from the bladder remained piliated, whereas organisms recovered from the kidney showed significantly less piliation (Schaeffer et al, 1987) (Fig. 10–6). (A to C, From Schaeffer AJ, Schwan WR, Hultgren SJ, Duncan JL. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect Immun 1987;55:373–80.) Studies in humans using indirect immunofluorescence of fresh urine bacteria have confirmed in-vivo expression and phase variation of pili. Analysis of the urine of adults with lower UTI detected type 1 pili in 31 of 41 specimens and P pili in 6 of 18 specimens (Kisielius et al, 1989). The piliation status of the bacterial population in the urine was heterogeneous, varying from predominantly piliated to a mixture of piliated and nonpiliated cells (Fig. 10–7). Strains isolated from different sites in the urogenital tract showed variation in the state of piliation. These results demonstrate that type 1 and P pili are expressed and subject to phase variation in vivo during acute UTIs. (A and B, From Kisielius PV, Schwan WR, Amundsen SK, et al. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect Immun 1989;57:1656.) This process of phase variation has obvious biologic and clinical implications. For example, the presence of type 1 pili may be advantageous to the bacteria for initially adhering to and colonizing the bladder mucosa. Subsequently, type 1 pili may be unnecessary for strains in suspension in urine and in fact detrimental because they enhance apoptosis, phagocytosis, and killing by neutrophils (Silverblatt et al, 1979; Mulvey et al, 1998). In the kidney, P pili may then take over as the primary mediator of bacterial attachment by means of their binding to the glycolipid receptors (Stapleton et al, 1995). The significance of epithelial cell receptivity in the pathogenesis of ascending UTI has been studied initially by examining adherence of E. coli to vaginal epithelial cells and uroepithelial cells collected from voided urine specimens. Fowler and Stamey (1977) established that certain indigenous microorganisms (e.g., lactobacilli, S. epidermidis) avidly attached themselves to washed epithelial cells in large numbers. When vaginal epithelial cells were collected from patients susceptible to reinfection and compared with such cells obtained from controls resistant to UTI, the E. coli strains that cause cystitis adhered much more avidly to the epithelial cells from the susceptible women. These studies established increased adherence of pathogenic bacteria to vaginal epithelial cells as the first demonstrable biologic difference that could be shown in women susceptible to UTI. Subsequently, Schaeffer and colleagues (1981) confirmed these vaginal differences in women, but in addition they observed that the increased bacterial adherence was also characteristic of buccal epithelial cells. As can be seen in Figure 10–8, there is a striking similarity in the ability of both cell types to bind to the same E. coli strain. In addition, there was a significant relationship between vaginal cell and buccal cell receptivity. Seventy-seven different E. coli strains were tested for their ability to bind to vaginal and buccal epithelial cells. A direct nonlinear relationship between buccal and vaginal adherence in controls and patients was confirmed for urinary, vaginal, and anal isolates. Thus high vaginal cell receptivity was associated with high buccal cell receptivity. (A and B, From Schaeffer AJ, Jones JM, Dunn JK. Association of in vitro Escherichia coli adherence to vaginal and buccal epithelial cells with susceptibility of women to recurrent urinary tract infections. N Engl J Med 1981;304:1062–6.) These observations emphasize that the increase in receptor sites for UPEC on epithelial cells from women with recurrent UTIs is not limited to the vagina and thus suggest that a genotypic trait for epithelial cell receptivity may be a major susceptibility factor in UTIs. This concept was extended by examining the human leukocyte antigens (HLAs), which are the major histocompatibility complex in humans and have been associated statistically with many diseases (Schaeffer et al, 1983). The A3 antigen was identified in 12 (34%) of the patients, which is significantly higher than the 8% frequency observed in healthy controls. Thus HLA-A3 may be associated with increased risk of recurrent UTIs. Reid and Sobel (1987) found that uropathogens attached in larger numbers to uroepithelial cells from women older than 65 years of age than to cells from premenopausal women 18 to 40 years of age. Raz and Stamm (1993) noted that susceptibility to recurrent UTI was increased by the lowered estrogen levels found in the postmenopausal women and that estrogen replacement decreased uropathogenic bacterial colonization and the incidence of UTI. Blood group antigens and carbohydrate structures bound to membrane lipids or proteins also constitute an important part of the uroepithelial cell membrane. The presence or absence of blood group determinants on the surface of uroepithelial cells may influence an individual’s susceptibility to a UTI. Sheinfeld and associates (1989) determined the blood group phenotypes in women with recurrent UTI and compared them with those of age-matched women controls. Women with Lewis Le(a−b−) and Le(a+b−) phenotypes had a significantly higher incidence of recurrent UTIs than women with Le(a−b+) phenotypes. There was no significant difference in the distribution of ABO or P blood group phenotypes. The Lewis antigen controls fucosylation. The protective effect in women with the Le(a−b+) phenotype may be due to fucosylated structures at the vaginal cell surface or in the overlying mucus, which decreases availability of putative receptors for E. coli (Navas et al, 1993). The nonsecretor status has also been associated with female acute uncomplicated pyelonephritis, especially in premenopausal women (Ishitoya et al, 2002). Stapleton and coworkers (1995) have shown that unique E. coli-binding glycerides are found in vaginal epithelial cells from nonsecretors but not from secretors. These studies individually and collectively support the concept that there is an increased epithelial receptivity for E. coli on the introital, urethral, and buccal mucosa that is characteristic of women susceptible to recurrent UTIs and may be a genotypic trait. The possibility that vaginal mucus might influence bacterial receptivity was investigated by Schaeffer and colleagues (1994). Type 1 piliated E. coli bound to all of the vaginal fluid specimens (Venegas et al, 1995). The binding capacity of vaginal fluid from women colonized with E. coli in vivo was greater than that from noncolonized women (Schaeffer, 1999). The importance of vaginal fluid in bacteria/epithelial cell interactions was investigated in an in-vitro model that measured the effect of vaginal fluid on the binding of bacteria to an epithelial cell line (Gaffney et al, 1995). Vaginal fluid from colonized women enhanced binding of bacteria to epithelial cells. Conversely, vaginal fluid from noncolonized women inhibited adherence. Thus the vaginal fluid appears to influence adherence to cells and, presumably, vaginal mucosal colonization. Subsequent studies demonstrated that secretory IgA is the primary glycoprotein responsible for vaginal fluid receptivity (Rajan et al, 1999). FimH binds mannosylated residues on the uroplakin molecules covering bladder superficial epithelial cells. The luminal surface of the bladder is lined by umbrella cells. The apical surfaces of umbrella cells appear as a quasi-crystalline array of hexagonal complexes composed of four integral membrane proteins known as uroplakins (Sun, 1996
Definitions
Incidence and Epidemiology
Pathogenesis
Routes of Infection
Ascending Route
Hematogenous Route
Urinary Pathogens
Fastidious Organisms
Anaerobes in the Urinary Tract
Mycobacterium tuberculosis and Other Nontuberculous Mycobacteria
Bacterial Virulence Factors
Early Events in UPEC Pathogenesis
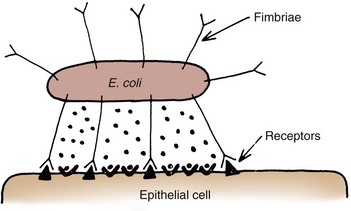
Bacterial Adherence
Bacterial Adhesins
Type 1 (Mannose Sensitive) Pili
Other Adhesins
Phase Variation of Bacterial Pili in Vivo
Epithelial Cell Receptivity
Vaginal Cells
Variation in Receptivity
Bladder Cells
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Infections of the Urinary Tract
A first or isolated infection is one that occurs in an individual who has never had a UTI or has one remote from a previous UTI.