Chapter 33 Infections and Infertility
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Several pathogens associated with STDs are known to cause infertility (Table 33-1) or other syndromes (Table 33-2). In Western society the most common of these pathogens are Neisseria gonorrhoeae and Chlamydia trachomatis. However, other pathogens may influence fertility as well.
Table 33-1 Sexually Transmitted Diseases
Table 33-2 Syndromes Associated with Curable Sexually Transmitted Diseases
| Cervical cancer |
Gonorrhea
Neisseria gonorrhoeae is a gram-negative coccus that grows in pairs, hence the term diplococcus. N. gonorrhoeae mainly infects columnar or cuboidal cells and not the squamous epithelium of a postpubertal girl. After attaching to the mucosal epithelium, it penetrates through the epithelial cells and into the submucosal tissues. Neutrophils respond and cause sloughing of the epithelium, submucosal microabscesses, and formation of pus. If left untreated, neutrophils are eventually replaced by macrophages and lymphocytes.
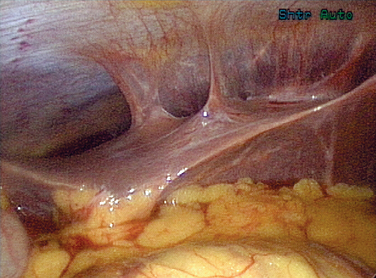
Gonorrhea can also pass beyond the fimbraie of the fallopian tubes and travel up the paracolic gutters and produce an infection around the liver. This is perihepatitis, or Fitz-Hugh–Curtis syndrome (Fig. 33-1). In unusual cases, gonorrhea can produce a disseminated disease with skin rash, infectious arthritis, and rarely endocarditis.
Treatment of a gonococcal infection depends on the location of the infection. If limited to the cervical region, the Centers for Disease Control and Prevention (CDC) recommends treatment with fluoroquinolones, ceftriaxone, or azithromycin (Table 33-3).
Table 33-3 Sexually Transmitted Disease Treatment Guidelines for Adults
| Chancroid | Azithromycin 1 g po × 1 or ceftriaxone 250 mg IM in a single dose or ciprofloxacin 500 mg po BID × 3 d or erythromycin base 500 mg po TID for 7 d |
| Herpes simplex virus — primary | Acyclovir 400 mg po TID for 7–10 d or acyclovir 200 mg po five times a day for 7–10 d or famciclovir 250 mg po TID for 7–10 d or valacyclovir 1 g po BID for 7–10 d |
| Herpes simplex virus recurrences | |
| Herpes simplex virus — suppression | Acyclovir 400 mg PO BID or famciclovir 250 mg po BID or valacyclovir 500 mg QD or Valacyclovir 1 g po QD |
| Syphilis — primary and secondary | Benzathine penicillin G 2.4 million units IM × 1 |
| Early latent syphilis | Benzathine penicillin G 2.4 million units IM × 1 |
| Late latent syphilis | Benzathine penicillin G 7.2 million units total, administered as three doses of 2.4 million units IM at 1-week intervals |
| Neurosyphilis | Aqueous crystalline penicillin G 18–24 million units per day, administered as 3–4 million units q4h for 10–14 d |
| Chlamydia cervicitis | Azithromycin 1 g po × 1 or doxycycline 100 mg po BID for 7 d |
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum | Ceftriaxone 125 mg IM × 1 or cefixime 400 mg po × 1 or ciprofloxacin* 500 mg po × 1 or ofloxacin* 400 mg po × 1 or levofloxacin* 250 mg po × 1 plus treatment for chlamydia if not ruled out |
| Bacterial vaginosis | Metronidazole 500 mg po BID × 7 d or metronidazole gel 0.75% one full applicator intravaginally qd × 5 d or clindamycin cream 2% one full applicator intravaginally qhs × 7 d |
| Trichomoniasis | Metronidazole 2 g po × 1 or tiridazole 2 g po × 1 |
| Human papillomavirus — external genital warts | Patient-applied: podofilox 0.5% solution or gel BID × 3 d then none for 4 d, repeated up to 4 cycles prn or imiquimod 5% cream qhs 3 × week for up to 16 wk |
| Human papillomavirus — external genital warts | Provider-applied: cryotherapy q 1–2 wk or podophyllin resin 10%–25% in tincture of benzoin q wk or trichloroacetic acid q wk or surgical removal |
* Should not be used in men who have sex with men or in those with a history of recent foreign travel or partners’ travel or infection acquired in California or Hawaii.1
Adapted from CDC Sexually Transmitted Diseases Treatment Guidelines 2006. MMWR 55:1–95, 2006.
Fluoroquinolone resistance in gonococci is becoming common in the Far East and Hawaii. Recently, it has begun emerging as a significant problem in some populations in the mainland United States. In men having sex with men, the prevalence of fluoroquinolone resistance has reached nearly 5%, prompting the CDC in 2004 to recommend that ofloxacin is not the drug of choice in this population.1 The resistance will continue to spread to other populations, and we will undoubtedly see further changes in the recommendations for the general population.
Chlamydia
The National Longitudinal Study of Adolescent Health (Wave III), using ligase chain reaction assays on first-void urine specimens, found chlamydia prevalence of 4% among young adults. The prevalence was greatest among minority women, including African Americans, Native Americans, and Latinos.2 The rate for young African American women was 6 times greater than for white women. From 1987 through 2002, the reported rate of chlamydial infections among all U.S. women increased nearly sixfold, from approximately 78 to 455 cases per 100,000 women. The etiology of this dramatic increase is likely multifactorial and includes increased screening, the use of the more sensitive nucleic acid amplification test (NAT), and better reporting, in addition to an increased infection rate.2
Cervical and upper genital tract chlamydial infections are treated with doxycycline, azithromycin, or erythromycin. The patient and all partners must be treated simultaneously. Treatment options are listed in Table 33-3.
Syphilis
The effect of syphilis on pregnancy has been well documented. Syphilis clearly produces miscarriages, prematurity, stillbirths, neonatal deaths, and congenital disease in the infant. The effects of syphilis on fertility are not clear, but a study out of Senegal did find that women age 40 or older with past or active syphilis were significantly more likely to have no history of gestation than women without evidence of syphilis infection.3
Although syphilis may have devastating long-term consequences if not treated, this disease is relatively easy to treat once diagnosed. Penicillin is the mainstay of therapy (see Table 33-3).
Trichomonas
Several studies have found that women with T. vaginalis infections had an increased risk of tubal infertility (up to 1.9-fold), and that this risk increased sixfold with more than four episodes of T. vaginalis infection.4–6 These organisms have been found in the abdominal cavity of women with acute salpingitis, and there is speculation as to whether or not the motile trichomonads are able to carry bacteria or viruses to the upper genital tract.7
Diagnosis is made most frequently using a wet mount prep of the vaginal discharge and visualizing the motile protozoa. Culture of the trichomonad is the gold standard test but is not widely used in clinical practice. A rapid test utilizing an immunochromatographic strip that can be performed rapidly in a clinical office is being evaluated for clinical use, with reported sensitivities near that of culture.
Treatment is with metronidazole 2 grams orally in a single dose. Tinidazole has also been approved in the United States for treatment of trichomoniasis in a 2-gram single oral dose, but is more costly (see Table 33-3).
Human Papillomavirus
Although HPV has not been shown to directly affect a woman’s fertility, lesions large enough to affect coitus are certainly problematic. The treatments required for localized preneoplastic or neoplastic lesions (i.e., cryotherapy, loop electrosurgical excision; see Table 33-3) can rarely cause scarring or affect the production of cervical mucosa, both of which could hinder the passage of sperm, thus affecting fertility. Invasive cervical neoplasias can require hysterectomy or other significant pelvic surgery.
Treatment does not eradicate the virus; thus prevention becomes especially important for this sexually transmitted infection. Recent advances have shown that a three-dose HPV-16 vaccine prevented the development of persistent HPV-16 infection and HPV-16-related cervical neoplasia in college age women for the first 17 months after vaccination.8 This finding will certainly lead to further vaccine developments for all of the major high-risk HPV types.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree