Linda Marie Dairiki Shortliffe, MD
Bacterial urinary tract infection (UTI) is the most common cause of childhood urinary tract inflammation. Beginning with bacterial adherence to host epithelial cells at the portal of entry, bacterial clonal studies now clearly support the fecal-perineal-urethral route and subsequent retrograde ascent of bacteria that cause UTI (Tullus et al, 1986; Mitsumori et al, 1997; Yamamoto et al, 1997). In UTI, both bacterial virulence factors and the human immune system (innate and adaptive) interact, and both are critical in understanding UTI pathogenesis. The practitioner must consider the human microbiome, moreover, as a community when managing difficult and recurrent UTI. We need to understand issues of (1) prenatal fetal anatomy, (2) issues of infection prevention versus treatment, and (3) medical globalism.
Epidemiology of Pediatric Urinary Tract Infections
Only during the first year of life do males get more UTIs than females (Asscher et al, 1973; Winberg et al, 1974), and during that year, the risk of UTI in uncircumcised boys is about ten times that of circumcised boys (Rushton and Majd, 1992a; Wiswell and Hachey, 1993). By age 1 year, 2.7% of boys and 0.7% of girls have had bacteriuria (Wettergren et al, 1980). Although this incidence declines to less than 1% in school-age boys (ranging from 0.03% to 1.2%), it rises to 1% to 3% in school-age girls (Asscher, 1975; Savage, 1975; Bailey, 1979), with sexually active females having more UTIs than sexually inactive ones (Kunin and McCormack, 1968).
Data from the U.S. Healthcare Cost and Utilization Project (HCUP) show that UTI causes about 51 per 100,000 children per year to be hospitalized and 174 per 100,000 infants (children younger than 3 months); about 18 to 20 per 100,000 of these cases are coded as pyelonephritis (Freedman, 2004). Overall, girls were hospitalized 2.5 times more than boys. UTI accounts for 2.4% to 2.8% of physician visits for children with commercial insurance or Medicaid; the National Ambulatory Medical Care Survey shows that UTIs are 0.7% of all pediatric office visits, thus documenting a heavy burden of childhood ambulatory UTI visits (Freedman, 2004). Given an average of 40,000 admissions for UTI per year (HCUP data) and a mean cost of $4500 per child for each UTI admission (Freedman, 2004), the financial cost for hospitalization of children with UTI alone is $180 million per year, without including ambulatory visits that account for much of UTI treatment in this country (Freedman, 2004).
Definition of Infection
Urinary collection is important for the reliability of UTI diagnosis (Fig. 116–1).
Classification of Urinary Infections
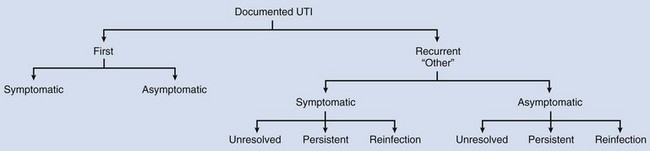
Recurrent infections may be (1) unresolved during initial therapy, (2) persistent (from an anatomic site) and/or (3) reinfection from a separate microorganism (Stamey, 1975). As will be discussed, first childhood UTIs are complicated because of evaluation and management implications and considerations.
UTI imaging evaluation reveals most sources of bacterial persistence early in childhood. The discovery of surgically correctable sources of bacterial persistence is obviously important so that these sites can be removed (Table 116–1). The majority of recurrent UTIs are not surgically correctable, because they result from reinfection from the same or a different gut microorganism. Screening urinary culture when a child is being examined for reasons unrelated to urinary symptoms, may diagnose an asymptomatic or covert UTI (Savage, 1975); these UTIs will be discussed.
Table 116–1 Surgically Correctable Causes of Bacterial Persistence in Children
Bacteria
Some bacteria appear to have a special affinity for causing UTI. The most common bacteria infecting the urinary tract are the gram-negative Enterobacteriaceae, usually Escherichia coli. Specific cell wall O-antigens that are identified by serotyping, such as E. coli serotypes 01, 02, 04, 06, 07, and 075, commonly cause pediatric UTI (Kunin et al, 1964; Bergström et al, 1972; Winberg et al, 1974), and many of these are now called UPEC (uropathogenic E. coli) or are included among ExPEC (extraintestinal E. coli) (Russo and Johnson, 2000). Experimental findings suggest that current Enterococcus faecalis with multidrug resistance may have a specific affinity for the kidney (Kau et al, 2005).
Bacterial surface structures, pili or fimbriae, may increase uropathogenicity. The bacterial fimbriae mediate bacterial adherence to uroepithelial cells and agglutinate red blood cells. Red blood cell agglutinating characteristics of E. coli, called hemagglutination, are blocked by different sugars (Duguid et al, 1978; Svanborg Edén and Hanson, 1978). Using this characteristic, Källenius and associates (1981) discovered that pyelonephritic E. coli cause mannose-resistant hemagglutination (MRHA) of human red blood cells. The terminal glycolipid of the human red cell P blood group antigen is a receptor that binds the P fimbriae on these E. coli. Therefore two important markers for E. coli virulence are MRHA characteristics and P blood group–specific adhesins (P fimbriae or P pili) (Källenius et al, 1981; Väisänen et al, 1981).
Most E. coli strains causing pediatric clinical pyelonephritis have both MRHA and P fimbriae (MRHA 91% [29/32], P fimbriae 81%) (Väisänen et al, 1981). P fimbriae are absent on less virulent strains. Källenius and associates (1981) found P fimbriae on 94% (33/35) of E. coli causing acute pyelonephritis, 19% (5/26) of E. coli causing acute cystitis, 14% (5/36) of E. coli causing asymptomatic bacteriuria, and on 7% (6/82) of E. coli from the feces of healthy children.
Two virulence factors appear to subvert the urinary tract immune system: one found in UPEC strains that acts by inhibiting Toll-like receptor (TLR) signaling (Cirl et al, 2008) and another that is a secreted autotransporter toxin (serine protease autotransporter) that damages tight junctions in epithelial cells (Guignot et al, 2007).
Other less well-characterized virulence factors are hydrophobic E. coli properties and the iron-binding capability of the bacteria associated with aerobactin production (Jacobson et al, 1988, 1989b). Genes with three specific uropathogenic proteins (USP, a Vibrio cholerae zot gene homologue; IrgA homologue adhesin Iha, a nonhemagglutinating adhesin; IroN(E. coli), a catechole siderophore receptor homologue) are associated with factors that cause UTI and pyelonephritis (Bauer et al, 2002).
Pathogenesis of Urinary Tract Infection in Children
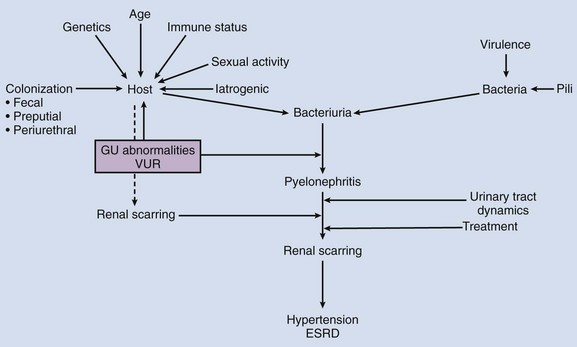
The natural course of UTI in children is unpredictable. Although risk factors and bacterial virulence influence this course, these factors alone are unhelpful in predicting who gets pyelonephritis, renal scarring, or parenchymal and functional loss from a single or recurrent UTI. Of the 3% of girls and 1% of boys who get a prepubertal UTI (Winberg et al, 1974), 17% or more will get infection-related renal scarring. Of those with scarring, 10% to 20% may become hypertensive, and only a rare child acquires progressive renal dysfunction culminating in end-stage renal disease. The course of UTI in adults and children differs, and studies on UTI in children are reported without regard for age and other child-specific factors (Shortliffe and McCue, 2002). For this reason, much of the course of pediatric UTI remains elusive.
Cystitis and Pyelonephritis
The bladder’s normal lack of susceptibility to bacteria is a complex interplay between bacterial virulence factors and the innate immune responses at the level of the uroepithelial cell. Because adaptive immune systems are incapable of immediate response to continual bacterial challenge at the urethral portal of entry, the initial response must be innate and reflexive for the host who is at times sitting in fecal microbiota (i.e., diapers). For this reason, the role of the fecal flora and human microbiome transitions—for reasons of diet, geography, pharmacotherapy, or epidemic and endemic contacts—must not be underestimated (Hällgren et al, 2005; Manges et al, 2008; Moreno et al, 2008; Smith et al, 2008) (Fig. 116–2).
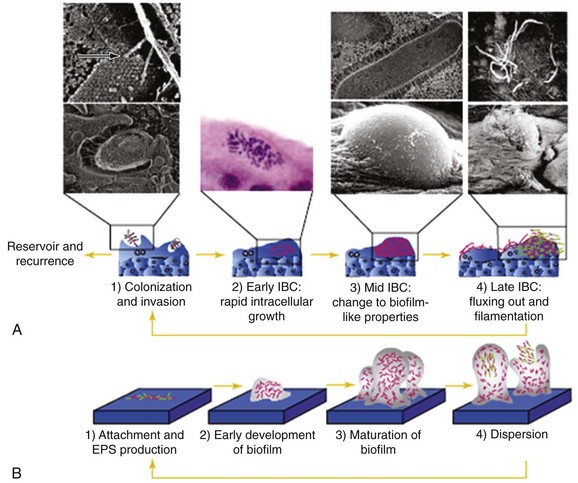
(From Kau A, Hunstad D, Hultgren SJ. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr Opin Microbiol 2005;8:54; Fig. 2.)
Bacterial genetic clonal studies confirm that entry into the urinary tract occurs through the suspected fecal-perineal-urethral route with subsequent retrograde ascent of periurethral bacteria (Kaijser and Larsson, 1982; Tullus et al, 1984; Mitsumori et al, 1997; Yamamoto et al, 1997). Bacteria overcome urethral defenses of urethral washout, epithelial shedding, and paraurethral glandular secretion, all of which vary by gender, age, and individual, causing variable likelihood for bacterial ascent and establishment of UTI (Kunin, 2002). At the molecular level, microbial polysaccharides may trigger urothelial receptors (Toll-like receptors) that activate the local immune system (Akira and Takeda, 2004). Tamm-Horsfall protein (THP) a common urinary protein, moreover, adheres specifically to type 1 fimbriated uropathogenic E. coli to participate in the host defense by helping to wash out bacteria during voiding (Bates et al, 2004; Raffi et al, 2005; Säemann et al, 2005) and may decrease inflammation during injury (El-Achkar et al, 2008).
Rodent and murine models have revealed the potential bacterial and uroepithelial interactions through which UTI occurs, and the models explain a new paradigm for acute and recurrent UTI. Superficial bladder umbrella cells are unique slow-cycling cells that renew only every few months. These cells contain special two-dimensional hexagonal crystals of 16-nm uroplakin particles that normally allow these superficial cells to flatten, expand, and allow normal bladder surface activity (Kong et al, 2004). A genetically altered uroplakin-deficient mouse has major urinary tract abnormalities.
Although the initial event in infection is attachment between the uroepithelial cell and bacteria through factors such as type 1 pili and P fimbriae, this attachment initiates a molecular interaction in which the adhesin-receptor complexes trigger a series of events that causes uptake of the bacteria into the bladder surface umbrella cells (Kau et al, 2005). In response, the host response rapidly sheds the uroepithelial cells (Mulvey et al, 1998). The bacteria then enter the bladder epithelial cells, interacting with lipid rafts and uroplakins and forming intracellular bacterial communities. These bacterial communities develop, and the organisms transform into an organized biofilm and have a more coccoid form. As these communities enlarge, they form cellular “pods” that protrude from the surface and may detach. These may then retransform into their rodlike motile form and disseminate through the urinary tract (Anderson et al, 2003). The intracellular bacterial “pod” may defend bacteria from normal host cellular immune and antimicrobial effects (Justice et al, 2004). These biofilms appear to allow microbial adaption to variable environments and then allow aggregate detachment that will cause systemic infection, antimicrobial resistance through plasmid exchange, endotoxin production, and overall increased resistance to host immune systems (Donlan, 2002). Simultaneously, however, the bacteria also attach to uroepithelial cell receptors (Toll-like receptors) and initiate a dramatic immune response with release and activation of proinflammatory cytokines and chemokines (IL-1, IL-6, IL-8) that signal and direct neutrophil transmigration across the epithelial barrier and into the urinary tract lumen, creating the clinical symptoms and signs of bladder and urinary tract infection. The balance between these processes determines whether UTI occurs.
Once bacteria are in the bladder, host immunity, impaired ureteral peristalsis, vesicoureteral reflux, and the specific organism uropathogenicity affect possible retrograde ascent. Under normal circumstances, nonspecific mechanisms, such as cytokine release, may play a role (Davidoff et al, 1997).
Pyelonephritis is important because of the clinical severity of the illness and the potential renal damage that may occur. Bacterial infection of the urinary tract also causes bladder and ureteral inflammation with tissue changes that alter dynamics of the entire urinary tract (Hinman, 1971; Boyarsky and Labay, 1972; Issa and Shortliffe, 1992; Johnson et al, 1992). Animal studies have shown that UTI causes abnormally elevated renal pelvic pressures even if (or especially if) vesicoureteral reflux is absent (Issa and Shortliffe, 1992; Angell et al, 1998), thus giving increased pressure and poor smooth muscle compliance as a further explanation for ureteral dilation observed in patients with acute pyelonephritis and otherwise normal upper collecting systems (Mårild et al, 1989).
Determining the point of clinical progression from cystitis to pyelonephritis or differentiating between these entities is impossible, because simple techniques with which to localize the level and extent of urinary tract bacteria are lacking. Although ureteral catheterization with sequential cultures is the gold standard for localizing upper and lower tract bacteria, this requires cystoscopy and is an impractical invasive way of following the course of infection (Stamey, 1980). Moreover, localizing the site of bacteria does not show the extent of renal inflammation. The Fairley bladder washout localization technique requires urethral catheterization during acute infection and subsequent bladder irrigation and washing with sterile water to localize bacteria to bladder (found in the first bladder culture) or above the bladder (found in cultures taken after the washing). Clinical symptoms correlate poorly with the results of localization studies using the Fairley or ureteral catheterization techniques. In one study comparing clinical symptoms with Fairley results, fewer than half the patients (mainly children) with fever and flank pain had upper tract bacteria (34/73) on Fairley test, and almost 20% (83/473) who were asymptomatic had upper tract bacteria (Busch and Huland, 1984).
Key Points
Urinary Tract Infection Pathogenesis
Risk Factors for Bacteriuria and Renal Damage
Although we are unable to alter bacterial pathogenicity at this time, it is worth reviewing characteristics that alter risk of UTI and renal damage and that may be therapeutically altered (Fig. 116–3).
Age
The prevalence of UTI is age dependent. Bacteriuria is more common at the extremes of life—in neonates and the elderly (Shortliffe and McCue, 2002). As already discussed, the prevalence of UTI for both males and females younger than 1 year is higher than at other times during childhood. There may be many factors causing this age-dependent relationship, some of which involve interactions with other host factors, such as periurethral colonization, breast-feeding, or immature immune status. Although none of these factors appears to be independent, the importance of the various factors may be age related. Others have discussed major factors creating risk for UTI in females, for instance, sexual intercourse, contraceptive usage (diaphragm/spermicidal), antimicrobial usage, previous history of UTI, estrogen status, and postvoid residual (Stamm and Raz, 1999). The degree to which these factors contribute is more or less dependent upon the subject’s age and previous history.
Genetics
Multiple host genetic factors that affect risk have been identified. Individuals who have childhood UTI continue to be at risk for adult UTI whether or not they have vesicoureteral reflux (Beetz et al, 2002). Obviously, issues related to gender, race, or ethnicity have specific genetic links and will be discussed separately.
Both alteration of bacterial–uroepithelial attachment related to bacterial virulence, which depends upon microorganism specificity, and alteration of the host immune response affect periurethral colonization. Children who originally had primary vesicoureteral reflux that resolved are commonly still susceptible to recurrent UTI, although symptoms are confined to the lower tract (Mansfield et al, 1995; Beetz et al, 2002). Clinical histories support a familial risk for UTI. On screening, sisters of girls who have recurrent UTI have a higher incidence of significant bacteriuria cultures than expected for the normal population (Fennell et al, 1977; Stauffer et al, 2004). Notably, two distinct risks for recurrent UTI in young women (age 18 to 30 years) are age at first UTI (less than age 15 years; odds ratio [OR] = 3.9) and UTI in the mother (OR = 2.3) (Scholes et al, 2000). The possible genetic relationship of renal scarring and UTI is discussed in the section Renal Scarring.
In the early 1980s, when bacterial P fimbriae were identified as a virulence factor, glycolipids characterizing the P blood group antigens were found on host uroepithelial cells and found to act as bacterial receptors. Because hosts with these receptors (i.e., P blood group phenotype) were suspected to have increased susceptibility for infection, children with recurrent infections were tested for this P blood group phenotype. The P1 blood group phenotype was found in 97% (35/36) of girls with recurrent pyelonephritis with minimal (grade I) or no reflux and was found in 75% (63/84) of controls without UTI (Lomberg et al, 1983). They found, moreover, that bacteria binding to glycolipid receptors (bacteria with P fimbriae) were more likely to be found in the girls with the P1 blood group without reflux than in those with the P1 blood group having reflux, suggesting that vesicoureteral reflux is more likely to contribute to ascent of the bacteria once UTI occurs than would the UTI itself.
Other blood group antigens on the urothelial surface (ABO, Lewis, and secretor phenotypes) influence UTI susceptibility. Adult women with Le (a−b−) and Le (a+b−) blood phenotypes are 3 times more likely to have a recurrent UTI than Le (a−b+) women, and epithelial cells from nonsecretors have more bacterial receptors than cells from secretors (Sheinfeld et al, 1989). Similarly, in children with UTI, the frequency of Le (a−b−) phenotype is higher, and the relative risk of infection in these children is 3.2 (Jantausch et al, 1994). Analysis of children with various anatomic abnormalities (e.g., ureteropelvic junction obstruction, vesicoureteral reflux, ureteroceles, and primary obstructive lesions) suggests that children with these abnormalities and a presumed nonsecretor phenotype (minimal or undetectable ABO and Leb immunoreactivity) are more likely to have had a history of UTI (16/17, 94.1%) (Sheinfeld et al, 1989, 1990). More recently, the nonsecretor status of ABH blood type antigens (ABH Ag) was found to be associated with 99mTc-dimercaptosuccinic acid (DMSA)–defined focal renal scarring in children with vesicoureteral reflux (Kanematsu et al, 2005).
Predisposition for pyelonephritis correlates with decreased expression of IL-8 receptors (CXCR1) on peripheral neutrophils (Frendéus et al, 2001; Lundstedt et al, 2007; Ragnarsdóttir et al, 2008) and polymorphisms of cytokine interleukin-8 (IL-8) regardless of vesicoureteral reflux status (Artifoni et al, 2007). Genetic polymorphisms, such as of intercellular adhesion molecule-1 (ICAM-1), which modulates the inflammatory infiltration into the surrounding tissues (Gbadegesin et al, 2006), and of transforming growth factor-β1 (TGF-β1) (Yim et al, 2007), which may affect other parts of the inflammatory and scarring process, are also associated with children who develop UTI-associated renal scarring. Other, separate gene polymorphisms of TLR correlate with UTI (TLR2 with both symptomatic and asymptomatic UTI [Tabel et al, 2007]; TLR4 with UTI and asymptomatic UTI [Karoly et al, 2007; Ragnarsdöttir et al, 2007] and vascular endothelial growth factor [VEGF] [Yim et al, 2007]).
Race and Ethnicity
Although UTIs occur in all races, epidemiologic studies show varying prevalence and complications of UTIs in different races. These factors are incompletely studied. In one comparative study of children less than 2 years old, including white, Hispanic, and African-American children, UTIs were most prevalent in white, then Hispanic, and finally African-American girls, but, in boys, UTIs were most common in Hispanic, white, and African-American boys, in that order. Circumcision status confounds these data (Bachur and Harper, 2001a, 2001b). Several studies show that African-Americans have fewer UTIs, lower incidence of vesicoureteral reflux, and perhaps less likelihood of reflux nephropathy than Hispanics or whites (Kunin and McCormack, 1968; Kunin, 1970; Lohr et al, 1994; Hoberman and Wald, 1997; Pinto, 2004). Interestingly, it has been reported that in Chinese children, UTI, vesicoureteral reflux (VUR), and renal scarring are more common in boys than girls; however, the ages are unreported, and these findings could be confounded by age and circumcision status (Howard et al, 2001; Williams et al, 2001a).
Renal Scarring
Renal scarring correlates with later UTI complications, such as hypertension, renal insufficiency, progressive renal scarring, and renal functional deterioration (Berg, 1992). Clinical studies that examine the association of recurrent bacteriuria and new renal scarring or progressive renal scarring support the notion that renal scarring begets renal scarring (Newcastle Asymptomatic Bacteriuria Research Group, 1975; Savage, 1975; Cardiff-Oxford Bacteriuria Study Group, 1978; Newcastle Covert Bacteriuria Research Group, 1981). Long-term follow-up has also shown that kidneys that were initially normal after UTI tend to remain normal, while those that were initially scarred appear to be more likely to suffer subsequent damage (Smellie, 1991; Smellie et al, 1998).
Angiotensin-converting enzyme (ACE) gene polymorphisms that are associated with increased renin-angiotensin activity are implicated in renal scarring. Increased DD genotype polymorphism is associated with increased risk of progressive renal failure in IgA nephropathy, diabetic nephropathy, autosomal-dominant polycystic kidney disease, focal glomerulosclerosis, and in children with congenital urologic abnormalities who have evidence of renal parenchymal damage and reflux nephropathy (Brock et al, 1997, 1998; Ozen et al, 1999). Genetic polymorphisms that relate to the immune response, such as those of ICAM-1, which modulates the inflammatory infiltration into the surrounding tissues (Gbadegesin et al, 2006), and of TGF-β1, which may affect other parts of the inflammatory and scarring process (Yim et al, 2007), are associated with children who develop UTI-associated renal scarring.
A systematic review and meta-analysis concluded that primary vesicoureteral reflux is a weak predictor of renal scarring in children hospitalized with UTI (Gordon et al, 2003). Using a definition of UTI as fever, positive urinary culture, and a DMSA scan showing photopenia during acute illness, another meta-analysis concluded that the rate of renal scarring after acute pyelonephritis is 36% to 41.6%, and those children with VUR have a 2.8 times greater risk of renal scarring (Faust et al, 2009). Although both meta-analyses found that children with VUR were more likely to have scarring than those without, the severity of illness differed and might account for the differing conclusions.
Colonization
Periurethral Colonization
The incidence of UTI in infants is higher during their first few weeks to months of life than at any subsequent time in the next few years. During this time, the periurethral area of healthy girls and boys is colonized massively with aerobic bacteria (especially E. coli, enterococci, and staphylococci) (Bollgren and Winberg, 1976b). This colonization decreases during the first year and is unusual in children who do not get recurrent infections after age 5 years. After this period, only those women and children who suffer repeated UTI remain more colonized by periurethral gram-negative bacteria than those who do not get infections (Stamey and Sexton, 1975; Bollgren and Winberg, 1976a, 1976b). Times and conditions of increased periurethral colonization are therefore associated with increased risk of UTI.
Preputial Skin
During the first few months of life, foreskin, periurethral, and preputial bacterial colonization and UTI appear to correlate. This association between the foreskin and neonatal UTI has caused controversy regarding the advantages and disadvantages of circumcision (Hallett et al, 1976). This has resulted in numerous editorials and recommendations by the American Academy of Pediatrics (King, 1982; Cunningham, 1986; Roberts, 1986; Lohr, 1989; Schoen et al, 1989; Winberg et al, 1989; Poland, 1990; Schoen, 1990; American Academy of Pediatrics [Task Force on Circumcision], 1999b), and more recently, the controversy has expanded to include sexually transmitted infection.
In a group of mainly uncircumcised normal boys, Bollgren and Winberg (1976b) documented that preputial aerobic bacterial colonization is highest during the first months after birth, decreases after 6 months, and is uncommon after age 5 years. Subsequently, Wiswell and associates (1988) compared the periurethral bacterial flora (using intraurethral and glanular cultures) during the first year of life in circumcised and uncircumcised boys and found that, for the first 6 months, periurethral uropathogenic organisms were cultured more frequently from the uncircumcised than circumcised boys. They and others concluded that the foreskin is responsible for this finding (Hallett et al, 1976; Glennon et al, 1988; Wiswell et al, 1988).
Although the reasons for neonatal bacterial colonization of the foreskin are multifactorial, including immature immune status, unusual nosocomial colonization, breast-feeding (Winberg et al, 1989), and other characteristics mediating bacterial adherence (Fussell et al, 1988), this high colonization is associated with a greater number of neonatal UTIs. In a series of retrospective reviews of 55 worldwide U.S. Army hospitals, Wiswell and Roscelli (1986) examined the incidence of UTI diagnosed by catheterization or suprapubic aspirate in male infants hospitalized during 1974 to 1983. They found that the incidence of infections in circumcised male infants was 0.11% (193/175,317); that in uncircumcised infants was 1.12% (468/41,799); and that in female infants was 0.57% (1164/205,212). Because these numbers are from hospitalized children in Hawaii, they may underestimate UTI; others report higher overall incidence of UTI in infant boys of 2.2% to 4.1%, with the majority (70% to 86%) occurring in uncircumcised infants (Wiswell et al, 1985; Schoen et al, 2000; Wiswell, 2000).
Uncircumcised male infants, moreover, have an increased relative risk of UTI of 3.12 when compared with circumcised boys, but this risk decreases from 3.7 at age 1 year to 3.0 at age 3 years (Wiswell and Hachey, 1993; To et al, 1998; Wiswell, 2000). The majority of infections occurred during the first 3 months of life. When the UTI incidence of the first and the final years of the study are compared, the total number of UTIs increased as the circumcision rate decreased, leading Wiswell to conclude that UTIs are more frequent in uncircumcised boys (Wiswell and Roscelli, 1986; Wiswell et al, 1987).
Using case-control methodology, circumcised boys in a population with a high rate of uncircumcised boys (Australia) were compared with a population of circumcised boys. Craig and associates (1996) concluded that circumcision was still associated with a lower rate of UTI before and past the first year of life. Although there may be difficulties with these data related to retrospective analysis and selection bias, periurethral colonization is associated with increased risk of UTI in girls and women also.
Others found no difference in pre- and postoperative rates of UTI whether or not circumcision is performed at the time of ureteroneocystotomies (Kwak et al, 2004). At this time, there is no way of predicting whether a male infant with a normal urinary tract who gets a UTI has a genetic or familial predisposition for infection. Because few infant boys who suffer a neonatal UTI risk a recurrent UTI after 1 year if they remain UTI free in the interim, prophylactic antibiotics for 6 months may be helpful in these boys.
There is some evidence that circumcision in hospitalized (neonatal intensive care) premature boys may decrease risk of recurrent UTI during the neonatal period (Cason et al, 2000). UTI treatment in uncircumcised infant boys also tends to be more expensive to treat, because these children are more likely to be hospitalized, especially during the first 3 months of life (Schoen et al, 2000). The cause for this is unknown, but it is reported that the organisms involved in these uncircumcised boys are more virulent and appear more similar to the strains causing pyelonephritis and urosepsis in adults (Bonacorsi et al, 2005). After meta-analysis, one group has calculated that circumcision decreases risk of UTI with an odds ratio of 0.13, yet UTI is rare enough in boys and common circumcision complications high enough so that only boys having recurrent UTI or high risk for recurrent UTI may benefit from circumcision (Singh-Grewal et al, 2005).
Although a full discussion of sexually transmitted infections (STI) are beyond the scope of this chapter, recent literature focuses upon a relationship between STI and circumcision status. A systematic meta-analysis of literature evaluating studies that examine sexually transmitted urethritis and genitourinary ulcerative diseases concluded that circumcised males may be more likely to acquire urethral STI (primarily Chlamydia), whereas uncircumcised males may be more likely to acquire ulcerative diseases (primarily chancroid) without a difference between rates of gonococcal infection (Van Howe, 2007). As might be expected, however, interstudy diversity makes definitive conclusions difficult. Other recent studies suggest that specific groups of circumcised males have lower rates of human papilloma viral penile and urethral infection and reduced HIV prevalence, but again, the conclusions relate to special populations and may not be generalizable (Auvert et al, 2009; Nielson et al, 2009; Sobngwi-Tambekou et al, 2009; Warner et al, 2009).
Current data do not support routine neonatal circumcision for UTI prevention in neonates or children, although some evidence exists that boys with recurrent UTI may benefit (American Academy of Pediatrics [Task Force on Circumcision], 1999b; Singh-Grewal et al, 2005). Although there appears to be evidence that specific STI may be increased in specific populations, STI (e.g., Chlamydia, gonorrhea, syphilis, nonspecific urethritis, genital herpes or warts) rates, when adjusted for socioeconomic status and sexual behavior, show no significant difference based on neonatal circumcision status in developed countries (Dickson et al, 2008). Opinions on this differ (Thiruchelvam and Cuckow, 2005). Some reports document slightly higher UTI incidence following ritual circumcision, which may reflect surgical technique (Liora et al, 2002).
Fecal Colonization
Because most UTIs result from fecal-perineal-urethral ascent of bacteria, fecal colonization is an important consideration. The human fecal flora is dependent upon the surrounding microbial ecology, native immunity, and microbial-altering drugs and foods. Studies showing that fecal colonization with specific pyelonephritic bacteria may occur in a neonatal nursery or hospital, with subsequent bacteriuria or pyelonephritis occurring several months later, emphasizing the importance of abnormal fecal colonization (Tullus et al, 1986). Bacterial clonal studies also associate fecal and urinary bacteria with community outbreaks (Manges et al, 2006, 2008; Smith et al, 2008) and show household members may share rectal flora (Johnson et al, 2008a).
The creation and selection of resistant organisms in the gut by antimicrobial usage is well recognized. Because the fecal flora is commonly responsible for perineal and periurethral colonization, the importance of responsible antimicrobial usage should not be underestimated. In one study, antibiotic treatment of school girls with phenoxymethylpenicillin for intercurrent nonbladder infections, usually otitis media, caused colonization and bacteriuria with strains more likely to be symptomatic (Hansson et al, 1989b).
Immune Status and Infancy
Compromised native immunity is associated with increased risk of infection. In children, the immune status varies with normal development, with transmission either natural or acquired (e.g., vaccination, breast-feeding, illness), and with congenital or acquired immunodeficiencies. UTIs are common in infants and children with immature immune systems. For instance, serum IgG is lowest from age 1 to 3 months (Robbins, 1972) when periurethral colonization is high in normal children. Secretory IgA is an important human immunoglobulin at the secretory and mucosal surface, and it is transferred to the newborn in colostrum if the child is breast-fed. Serum IgA is found in diminished concentrations for the first several months and is either absent or almost absent at the secretory surfaces of the nasopharynx, gut, and urothelium during this period (Svanborg Edén et al, 1985; Fliedner et al, 1986; Yoder and Polin, 1986) and undetectable in the urine at birth. Urinary secretory IgA and total IgA increase during the first year and are higher in children who are breast-fed (James-Ellison et al, 1997). The role that urinary IgA plays in childhood UTI has not been investigated extensively. In children with acute UTI or pyelonephritis, urinary excretion of IgA is higher than in those without UTI, yet levels are exceeded in normal adults (Svanborg Edén et al, 1985; James-Ellison et al, 1997).
Urinary IgA in infants, moreover, is in the monomeric rather than the secretory form normally found in adults, suggesting that urinary IgA in infants may be systemic immunoglobulin excreted as a result of infection-related tubular dysfunction rather than secretory. Children with recurrent UTI have lower urinary secretory IgA concentrations than those without (Fliedner et al, 1986). The benefits of breast milk are unclear (Hanson, 1998; Committee on Breast Feeding, 2005). Case-control studies suggest that breast-feeding confers a protective effect against UTI during the first 6 months of life. These studies show the duration of breast-feeding is shorter in children who have UTI (9 vs. 16 weeks in children who had exclusive breast-feeding) (Mårild et al, 1989), but that full or partial breast-feeding may confer a protective effect against UTI for the first 6 months of life (Pisacane et al, 1990, 1992). Although the mechanism of the protection is unknown, the oligosaccharide content of the mother’s breast milk and urine are the same as in the breast-fed infant. Some hypothesize that the oligosaccharides inhibit the adherence of pathogenic E. coli to the uroepithelium (Coppa et al, 1990). Other studies show that exclusive breast-feeding for 4 or more months protects against single or recurrent otitis media in children or infants with cleft palate (Duncan et al, 1993; Paradise et al, 1994).
As expected, children with specific immune deficiency syndromes have altered immunity and commonly have an increased risk of infection and its dissemination. About 20% of human immunodeficiency virus (HIV)-infected children have bacterial UTI with both common and opportunistic organisms (Grattan-Smith et al, 1992), and others have associated UTI and HIV; specifically, in HIV-seropositive women, UTI was associated with high viral load (OR = 1.82) (Park et al, 2002). Specific deficiencies of the immune response associated with genetic polymorphisms that involve the TLR and cytokine-associated responses are discussed in the section Genetics.
Sexual Activity
Epidemiologic studies show that sexual activity is a risk factor for UTI. Sexually active females have more UTIs than inactive females (e.g., nuns) (Kunin and McCormack, 1968). This association has caused some to suggest that UTI is a marker for teenage sexual activity (Nguyen and Weir, 2002). Whether this relationship stems from colonization or other factors is unclear.
Genitourinary Anatomic Abnormalities
UTI has been a marker for pediatric genitourinary tract anatomic abnormalities; hence, the reason UTI evaluation consists of imaging. This maxim was reaffirmed when investigators followed children with prenatally detected renal pelvic dilation of 5 mm or greater at 28 weeks’ gestation, and found the children had increased risk of UTI (Coelho et al, 2008). Genitourinary abnormalities, in particular, nonfunctioning segments can serve as niduses of infection causing bacterial persistence because of renal inability to achieve urinary antimicrobial concentrations adequate to eradicate bacteria in these segments. Similarly, genitourinary partial obstruction or renal functional impairment may create increased risk of renal damage because of poor or inadequate antimicrobial penetration. A sequela of chronic UTI and inflammation, xanthogranulomatous pyelonephritis, may also occur in children with obstruction and UTI in renal segments (Stamey et al, 1977; Zafaranloo et al, 1990; Cousins et al, 1994; Hammadeh et al, 1994).
Vesicoureteral Reflux
Vesicoureteral reflux is discussed in Chapter 122. Reflux is common in children with UTI, but no correlation between reflux and susceptibility to UTI is evident (Winberg et al, 1974). At least one group has shown no difference in occurrence of recurrent UTI between children who have reflux and those who do not (Garin et al, 2006). Surveys show that 21% to 57% of children who have bacteriuria have vesicoureteral reflux when evaluated (Kunin et al, 1964; Abbott, 1972; Asscher et al, 1973; Newcastle Asymptomatic Bacteriuria Research Group, 1975). Although vesicoureteral reflux raises risk of pyelonephritis associated with bacteriuria, it correlates poorly with UTI-induced renal scarring (Gordon et al, 2003).
Peripubertal Girls with Persistent Vesicoureteral Reflux (Pregnancy)
The question of whether girls approaching puberty who have persistent vesicoureteral reflux should have their reflux corrected because of the potential for future pregnancy is problematic. If primary vesicoureteral reflux persists as a girl approaches puberty, it becomes statistically less likely each year to resolve spontaneously. For this reason, some advocate that all girls approaching puberty undergo correction of reflux to avoid the risks of pyelonephritis during future pregnancies. Whether a female with persistent vesicoureteral reflux who has never had a UTI and demonstrates no increased susceptibility for UTI should undergo routine correction of reflux is controversial; moreover, whether a female with persistent vesicoureteral reflux who has demonstrated increased susceptibility for UTI should undergo correction is also controversial. Although the prevalence of bacteriuria in pregnant women is the same as in nonpregnant women (Shortliffe and Stamey, 1986b; Shortliffe, 1991), the likelihood that bacteriuria may progress to pyelonephritis is greatly increased. Thirteen and a half to 65 percent of pregnant women who are bacteriuric on a screening urinary culture will develop subsequent pyelonephritis during pregnancy if untreated (Sweet, 1977), whereas pyelonephritis is rarely the consequence of uncomplicated cystitis in a nonpregnant woman.
The reasons for this increased likelihood of pyelonephritis during pregnancy are unknown, but they may relate to hormonal and hydrodynamic changes of pregnancy. There is increased bladder and urinary tract compliance and bladder enlargement, and an enlarging uterus may cause anatomic displacement of the bladder and ureters (Hsia and Shortliffe, 1995). There are no data to suggest that pregnancy causes vesicoureteral reflux (Shortliffe and Stamey, 1986b). When 321 women had a single-film voiding cystourethrogram during the third trimester of pregnancy or immediately postpartum, only 9 (2.8%) had vesicoureteral reflux (Heidrick et al, 1967). Although 6.2% (20/321) of these women had asymptomatic bacteriuria, only 1 of these 20 women had vesicoureteral reflux. Therefore in contrast to the pediatric situation, few pregnant women who develop pyelonephritis have reflux. Of the 9 women who had reflux, 3 developed pyelonephritis during a pregnancy.
In another series, 21 of 100 pregnant women (27 refluxing renal units in 21 patients) with asymptomatic bacteriuria evaluated postpartum had vesicoureteral reflux, mostly of low grade (67% into the ureter only), and 17% of these women had renal scarring (Williams et al, 1968). Although some use these data to justify correction of all pubertal reflux in girls, this renal scarring is unlikely to be the result of UTI during pregnancy. This rate of scarring is similar to that found in children with screening bacteriuria and renal scarring (Asscher et al, 1973; Newcastle Asymptomatic Bacteriuria Research Group, 1975; Savage, 1975). Because these women had no imaging before pregnancy, it is most likely that any renal scarring found post partum was present beforehand and occurred in childhood.
These data suggest that postpubertal females who have vesicoureteral reflux and a predisposition for frequent urinary tract infections will continue to have this predisposition for infections into adulthood and pregnancy. Moreover, those women who were susceptible to UTI before pregnancy will continue to be more likely to develop UTI during and after pregnancy, whether or not they have vesicoureteral reflux (Mansfield et al, 1995; Bukowski et al, 1998; Beetz et al, 2002).
Pregnant Women with Renal Insufficiency
When a practitioner counsels a woman with known reflux nephropathy and renal insufficiency about pregnancy, renal function needs evaluation. With physiologic pregnancy—renal hyperperfusion and resulting hyperfiltration—a blood urea nitrogen (BUN) greater than 14 mg/dL and serum creatinine greater than 0.9/dL may indicate pre-existing renal insufficiency (Vidaeff et al, 2008). Though outcomes data for pregnancy are sparse, when there is moderate or severe renal insufficiency (moderate, initial serum creatinine, 1.4 to 2.4 mg/dL; severe, 2.5 mg/dL or greater), even though infant survival may be greater than 90%, maternal and obstetrical complications are high (Bear, 1976; Davison and Lindheimer, 1978; Jones and Hayslett, 1996). In these high-risk women, 43% had a pregnancy-related deterioration in creatinine clearance of at least 25% that occurred during pregnancy (20%) or immediately postpartum (23%). In the majority, this pregnancy-related deterioration in renal function was irreversible (Epstein, 1996; Jones and Hayslett, 1996). This accelerated renal insufficiency was associated with increased hypertension and high-grade proteinuria. Almost 60% of these pregnancies ended in preterm delivery with cesarian section, and the majority of infants were below the 50th percentile in birth weight (Jones and Hayslett, 1996). These data have been interpreted to indicate that when the serum creatinine exceeds 2.0 mg/dL in a pregnant woman, she has a 1 in 3 risk of progressing to end-stage renal disease either during or just after delivery (Epstein, 1996).
The effect of a maternal UTI on the fetus has been controversial. Analyses of National Collaborative Perinatal Project (NCCP) data from the 1970s and Medicaid data from the 1990s (both representative of women of low socioeconomic status) show an association between third-trimester maternal UTI and fetal mental retardation or developmental delay; and NCPP data, furthermore, associates third-trimester UTI with increased chance of fetal death (McDermott et al, 2001). Others have documented infant low birth weight and prematurity (Naeye, 1979). With mild to moderate renal insufficiency, fetal survival is only slightly diminished (Vidaeff et al, 2008).
Neurogenic and Bowel-Augmented Bladders
Children who have neurogenic bladders with abnormally elevated bladder pressures risk increased renal damage. Risk of UTI is also increased secondary to increased instrumentation common with the neurogenic bladder (intermittent catheterization). Increased pressure within the neurogenic system combined with the physiologic effects of UTI that create increased pressure creates a greater likelihood for subsequent renal parenchymal damage (Hansen et al, 2003). Chronically or intermittently elevated bladder pressures may cause secondary vesicoureteral reflux from decompensation of the ureterovesical junction from the elevated pressure (Hutch, 1952). If this does not occur, the elevated bladder pressures associated with a neurogenic bladder may also cause effective ureterovesical obstruction. This obstruction increases the risk of renal damage associated with urinary tract infections (Cowan and Shortliffe, 1998), and as discussed earlier in this chapter, intermittent elevations in bladder pressure associated with physiologic bladder dynamics in the immature bladder may exacerbate the effects of vesicoureteral reflux and UTI.
Forty to 80 percent of the urinary specimens sampled had bacteriuria and/or pyuria (Taylor et al, 1985; de la Hunt et al, 1989; Joseph et al, 1989; Gribble and Puterman, 1993; Johnson et al, 1994; Schlager et al, 1995, 1998), and most were asymptomatic (Geraniotis et al, 1988; Joseph et al, 1989; Schlager et al, 1995). However, this catheter-associated asymptomatic bacteriuria appears to lack morbidity much of the time. Notably, even though a majority of these children have asymptomatic bacteriuria, most may undergo urodynamic studies without complications (Shekarriz et al, 1999).
Even though prophylactic antibiotics administered during clean intermittent catheterization may delay and possibly decrease bacteriuria in the short term (Johnson et al, 1994), long term there appears to be no proven efficacy and may cause increased microorganism resistance (Clarke et al, 2005). Meta-analysis of data on the use of sterile versus nonsterile, single versus multiple use, and lubricated versus nonlubricated catheters document no benefits among the alternatives in reducing UTI (Schlager et al 2001; Moore et al, 2007). Thus neither sterile, single-use lubricated catheters nor antimicrobial prophylaxis is recommended for long-term bladder management. The natural history of clean intermittent catheterization programs deserves further study.
Novel management for those with neurogenic bladders who acquire frequent symptomatic recurrent UTI is to induce asymptomatic UTI using previously identified strains from individuals with asymptomatic UTI (Trautner et al, 2008; Prasad et al, 2009). These studies are preliminary at this time.
Little investigation of the bacteriuria of bowel-augmented bladders exists. In a prospective study, those who have bladder substitution were found to have greater bacteriuria than those without, and there was a substantial local IgA antibody response to the resident bacteria and increased IgG response from the bladder or upper tracts (Iwakiri et al, 2002). Whether this local response contributes to asymptomatic UTI or is protective is unknown.
Iatrogenic Factors
The urinary tract is the most common site of nosocomial infection. Although there are no good data on risk of catheter-induced infections in children, in adult women the incidence of catheter-induced UTI ranges from 1% to 20% depending upon the circumstances of catheterization (Stamey, 1980). It has been documented that nosocomial UTI frequently complicates hospitalization in children, especially when urethral catheterization is performed (Dele Davies et al, 1992; Lohr et al, 1994), and this may be a reason to consider giving antimicrobial prophylaxis. On this basis, children who have hospital-acquired UTI, have urinary tract abnormalities, have recently been instrumented, or have had recent antimicrobial treatment are more likely to have infections caused by unusual and more antibiotic-resistant organisms (Ashkenazi et al, 1991).
When urethral catheters remain indwelling for longer than 4 days, bacteriuria is almost inevitable, because bacteria attach to the device surface and form biofilms of extracellular polymers, making these bound organisms resistant to routine antimicrobial treatment (Donlan, 2001). Because the urinary tract is second only to the mouth as the source of bacterial endocarditis, the American Heart Association recommends bacterial endocarditis antimicrobial prophylaxis before urethral catheterization in individuals with a known urinary infection. Cost-outcome analysis for bacterial endocarditis prophylaxis of febrile children with moderate cardiac lesions in an emergency room being evaluated for UTI shows no cost-benefit value (Caviness et al, 2004).
Endocarditis antimicrobial prophylaxis is not recommended, however, when urine is sterile. It is noteworthy that the British Society for Antimicrobial Chemotherapy revised their guidelines in 2006 and continue to recommend prophylaxis for prevention of enterococcal endocarditis for cystoscopy, urethral dilation, and transurethral procedures, whereas the American Heart Association in 2007 no longer recommended routine prophylaxis for gastrointestinal or urologic procedures with “uninfected tissues” (Pediatric Care Online, 2007; Wilson et al, 2007; Shanson, 2008).
In school-age boys, urethral self-manipulation with water injection or self-instrumentation can cause UTI (Labbé, 1990). About 25% of children who are victims of sexual abuse may complain of dysuria and urinary frequency, but these children rarely have UTI (Klevan and DeJong, 1990).
Key Points
Urinary Tract Infection Pathogenesis and Risk Factors
Diagnosis of Urinary Tract Infection
Rapid diagnosis of UTI is essential to initiating rapid antimicrobial treatment and preventing renal damage. In children who have no alternative fever source from history or physical findings, UTI accounts for greater than 5% of fevers (American Academy of Pediatrics, 1999a). In febrile infants (younger than 8 weeks), UTIs account for 13.6% of these fevers with the majority occurring in males (Lin et al, 2000). Young children may show only signs of generalized illness—fever, irritability, poor feeding, vomiting, and diarrhea. In a busy children’s hospital emergency room, the overall prevalence of UTI in febrile infants younger than 12 months and girls younger than 2 years with fever greater than 38.5° C and without other cause was 3.3%, with higher prevalence in whites, girls, and uncircumcised boys; white girls had a UTI prevalence of 16.1% (Shaw et al, 1998a, 1998b).
Symptoms
UTI is a common cause of pediatric bacterial infection (Crain and Gershel, 1990; Bonadio et al, 1993; Hoberman et al, 1993). Fever accounts for about 20% of pediatric office visits (Eggli and Tulchinsky, 1993), and UTI causes 4.1% to 7.5% of these febrile episodes (American Academy of Pediatrics, 1999a).
Infants
In seriously ill infants and young children, one must suspect UTI and obtain a urinary specimen, even if signs may point elsewhere. Especially in febrile infants, from birth to 8 to 10 weeks, neither clinical symptoms nor laboratory tests will predict a presumptive UTI. Even if clinical symptoms suggest other sites of infection, this does not eliminate the likelihood of a UTI (Crain and Gershel, 1990).
The prevalence of UTI in febrile infants (younger than 8 weeks) is about 13.6%, with the majority occurring in males (Lin et al, 2000). It is important to note that febrile infants not suspected of having a urinary source of infection are as likely of having a urinary source as those who are suspected of having a urinary source (5.1% vs. 5.9%). In infants with another possible fever source, such as otitis media, 3.5% also had a UTI (Hoberman et al, 1993).
Young Children
As in infants, children less than 2 years old have UTI symptoms that are vague and generalized—fever, irritability, poor feeding, vomiting, diarrhea, and ill appearance (Ginsburg and McCracken, 1982) (Tables 116-2 and 116-3). If one uses a clinical decision rule to decide which febrile young girls should be cultured for UTI, the presence of two of the following five variables may be useful: (1) age less than 12 months, (2) white race, (3) absence of other fever source, (4) fever greater than 39° C, and (5) fever of 2 days or more. Using these parameters, one can predict UTI with a sensitivity of 95% and specificity of 0.31 while avoiding 30% of unnecessary cultures (Gorelick and Shaw, 2000).
Table 116–2 Host Factors Affecting Bacteriuria
Table 116–3 Symptoms of Urinary Tract Infection in 100 Infants with Acute Urinary Tract Infections
| SYMPTOM | PERCENTAGE |
|---|---|
| Fever | 67 |
| ≥38° C | 100 |
| ≥39° C | 57 |
| Irritable | 55 |
| Poor feeding | 38 |
| Vomiting | 36 |
| Diarrhea | 36 |
| Abdominal distention | 8 |
| Jaundice | 7 |
Modified from Ginsburg CM, McCracken GHJ. Urinary tract infections in young infants. Pediatrics 1982;69:409–12.
Adolescents
Adolescents with lower urinary tract symptoms should not be treated as large children or young adults. Although E. coli is still the most common UTI in female adolescents, the second most common is Staphylococcus saprophyticus, and it may be associated with sexual activity and recurrent UTI (Huppert et al, 2007). In adolescent males, UTI may be associated with structural abnormalities and lower urinary tract symptoms with voiding dysfunction (unfortunately labeled prostatitis IIIb—noninflammatory, nonbacterial) (Li et al, 2006). Adolescent females with dysuria and frequency should be evaluated for both UTI and sexually transmitted infection (STI). There are estimates that 50% of high school students have had at least one sexual encounter. The prevalence of Chlamydia is 13% to 26% and Neisseria gonorrhea is about 2% to 10% in this population. For this reason, considering STI in this group (age 16 to 24 years) may be just as important, or more important, in considering UTI for the differential diagnosis (Claudius, 2000; Pimenta et al, 2000). A suggested marker for teenage sexual activity is UTI (Nguyen and Weir, 2002).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree