Author
N
Primary procedure (s)
Revisional procedure (s)
Follow-up duration (range)
Complications
Leaks
30-day mortality
Preoperative BMI (at primary procedure)
Pre-revision BMI
Pre-revision weight loss
Post-revision weight loss
Interval from primary operation–revision
Thompson, et al. (35) 2013
TORe (n = 50) or sham procedure (n = 27)
RYGB
Endoscopic sutured transoral outlet reduction (TORe)
6 months
1 pulmonary edema immediately post-procedure
0
37.6 ± 4.9 in –TORe group 38.6 ± 6.2 in control group
73.2 ± 20.5 in –TORe group (n = 50) 73.7 ± 21.5 in control group (n = 26)
15.9 ± 20.90 in TORe group (n = 43) 7.7 ± 20.18 in Control group (n = 26)
58.8 ± 25.7 months (n = 48) in TORe group 67.5 ± 24.5 (n = 27) in control group
Leitman, et al. (36) 2010
64
RYGB
Endoscopic plication and revision of the gastric pouch (EPRGP)
5.8 (3–12) months
2 (3 %) intraoperative complications (equipment failure), 1 observed for bleed (no transfusion)
0
48.5
39.5
nadir BMI 31
7.3 kg (0–31)
5 years
Himpens, et al. (37) 2012
88
LRYGB (with and without prior VBG or AGB)
Distal RYGB, Fobi ring around pouch, bypass reconstruction, LSG, plication
48 (18–122) months
Overall reoperation rate: 7.3 %, overall severe complication rate: 20.7 %, overall leak rate 12.1 %
12.10 %
0
42.7 ± 19.7 (33.0–56.6)
39.1 ± 11.3 (30.8–51.8)
12.4 ± 9.3 % (−1.0–29.1)
Post-revision BMI 29.6 ± 12.4 (18.0–45.5)
3.0 years (1.5–8.0)
Irani, et al. (43) 2011
43
RYGB
Salvage banding
26 ± 14 (6–66) months
12 adverse events: 1 enterotomy requiring band removal; 1 SBO, 1 GI bleed, 3 esophageal dilations resolved with band deflation, 1 minor port leak, 1 port flip, 1 band slip, 1 case of persistent dysphagia, and 2 cases of intragastric band migration
0
50.4 (35–60)
43.3 (34–60)
17 % EWL
Post-LAGB BMI: 33.8 (25–47); 38 % EWL from LAGB; 55 % cumulative (initial + revisional) EWL
223 ± 154 months
Rawlins et al. (45) 2011
29
RYGB
distal RYGB
1–5 years
Short-term: 0 leaks, 4 DVTs, 10 SSIs; Long-term: 1 partial SBO, 6 ventral incisional hernias, 9 w/albumin <3, 6 required TPN, 1 reversed
0
0
57.9 (38–81)
48.1 (35–67)
26.6 % (0–46 %) EWL
60.9 % (39–83 %) EWL at 1 year; 68.8 % (53–91 %) EWL at 5 years
Parikh et al. (59) 2007
12
RYGB
BPD-DS
11 (2–37) months
6 (4 strictures, 1 metabolic acidosis, 1 wound complication)
0
0
53.9 (40.7–66.0)
40.7 (33.2–46.0)
42 % (8–63 %) EWL; lowest BMI after primary RYGB: 31.6 (23.3–39.0)
62.7 % (18.8–96.2 %) EWL at 11 months 79.4 % (48.3–98.1 %) overall
NR
Dapri et al. (60) 2011
4
RYGB
LSG
11 ± 12.8 months
1 GG fistula
NR
0
43.2 ± 8
37.3 ± 6.6
27.5 ± 11.8 % EWL; 26.5 ± 12 % EBMIL
59.3 ± 31.5 % EWL; 42.3 ± 34.5 % EBMIL
36.7 ± 15.6 months
Endoscopic management to augment gastric restriction by reducing the pouch and gastrojejunal stomal size is a safe corrective procedure, and has been shown not only to arrest weight gain [35], but also attain short-term weight loss [36–38]. However, the published studies are mostly small non-controlled series and numerous devices utilized for this approach are commercially unavailable.
Indications for surgical revision of the pouch or gastrojejunostomy include significant pouch or stoma dilatation (Fig. 19.1), presence of gastrogastric fistula with inadequate weight loss or persistence of marginal ulceration [39, 40]. Various definitions have been used to define a “dilated” or large gastric pouch or gastrojejunostomy. While it is still unclear what the definition should be, in our practice we define a pouch larger than 5×5 cm or containing a large amount of fundus to be enlarged. We consider a gastrojejunostomy more than 2 cm in greatest diameter to be large as well, but these are arbitrary cutoffs and need to be placed in the context of the patient’s clinical course and overall evaluation. Since anatomic evaluations are not routinely performed for patients who are maintaining their weight loss, it is unclear how many patients with a “large” pouch or stoma by these criteria are able to achieve long-term success and this needs further study.
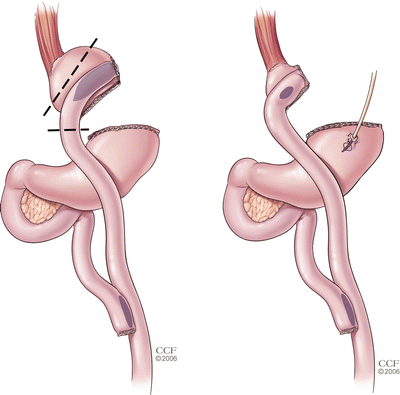

Fig. 19.1
Revision of a large gastric pouch or dilated gastrojejunostomy for weight gain after gastric bypass can be achieved with resection of the gastrojejunal complex and dilated pouch. A new, smaller gastrojejunostomy is then created. A gastrostomy tube can be placed in the gastric remnant at the surgeon’s discretion
Another corrective option is surgical placement of an adjustable or nonadjustable band around a gastric pouch to add additional gastric restriction [41–43]. While this has been shown to be a safe option, the utility of this type of adjunctive treatment is not clear. Like primary gastric banding procedures, there is considerable variability in reported outcomes utilizing the adjustable gastric band for additional weight loss after gastric bypass.
Other corrective surgical options include lengthening of the biliopancreatic limb to increase the malabsorptive component, or lengthening of the Roux limb to increase the bypass component. Duodenal switch as a conversion procedure for patients with inadequate weight loss after gastric bypass has been reported but is technically challenging and not widely accepted due to the risk associated with this conversion procedure. Currently, there are only feasibility data in the literature regarding this approach and no data regarding the long-term risks and benefits [44].
Improved weight loss after reoperative surgery has been reported by many authors, but the current evidence to support these strategies is limited to mostly single institution retrospective case series [45]. The lack of prospective data and the heterogeneity of the published data for revisional bariatric surgery can be partially attributed to the difficulties in getting access to care for these patients. Since many patients do not have coverage for revisional bariatric procedures or have limited options for reoperative surgery, there are relatively few large study cohorts in the literature. This is in stark contrast to available coverage for reoperative surgery provided by major national plans and state employee health plans for other surgical specialties (orthopedics, cardiac surgery) [46].
19.2 Sleeve Gastrectomy
19.2.1 Epidemiology and Etiology
Laparoscopic sleeve gastrectomy is still relatively new to bariatric surgery and has been widely utilized as a primary procedure for about 10 years. There is a growing body of long-term weight loss data in the literature but the true incidence of inadequate weight loss or weight regain after LSG is still not clear. In a study by Cesana et al., about 5 % of LSG patients required reoperation with a mean follow-up 21.1 ± 9.7 months (range 6–57 months) [47]. In another study, weight regain of 10 kg from nadir was observed in 19.2 %, i.e., in 5 of the 26 patients during the 5 year follow-up. In the weight regain group, the first year %EWL was comparable to the adequate weight loss group, however the %EWL significantly decreased by the second year in the weight regain group [48]. Like RYGB, the etiology of weight regain after LSG is multifactorial and likely involves anatomic, behavioral, socioeconomic, and psychological components. There are currently few published data that can help identify the right patient for the right bariatric operation, so bariatric surgeons rely on experience, clinical judgment, and patient preference to drive these decisions.
Dilation of the gastric lumen, particularly the gastric fundus, is a common imaging finding in patients with weight regain after LSG. This may be attributed to a lack of adequate calibration at the time of the primary procedure or a natural process of stomach tissue to dilate and become more compliant over time. Patients’ behavioral issues, eating habits, and lack of adherence to the post-surgical diet recommendations may also contribute to this problem [47].
19.2.2 Medical Management
As with RYGB, some patients may benefit from continued medical therapy after LSG. While hunger often disappears for several months after LSG, it inevitably returns and some patients may benefit from medication to control appetite long-term. With a variety of FDA-approved medication for the treatment of obesity available in the USA, these may play an important adjunctive role in the long-term management of some sleeve gastrectomy patients. Further research is necessary to better define the role of medical therapy for patients with IWL or weight regain after sleeve.
19.2.3 Surgical Management
A subset of patients with IWL after sleeve gastrectomy may benefit from additional surgical therapy if their weight loss or comorbidity improvement is suboptimal. In a recently published report by Sieber et al., 8 of 68 patients (11.8 %) underwent reoperative surgery due to IWL after sleeve gastrectomy [49]. However, similar to any bariatric procedure, the patient must be evaluated by a multidisciplinary team to determine the cause of weight regain. Surgical options include placement of an adjustable band over the proximal sleeve, re-sleeve gastrectomy (corrective), or conversion to gastric bypass or duodenal switch.
In one study of patients who had a LSG over a 60 French Bougie , placement of an adjustable band due to inadequate weight loss after sleeve gastrectomy resulted in a 78 lb weight loss within 9 months, corresponding to an EWL of 57 % [50]. Overall, though, there are not strong data to support this approach and it is not commonly used.
In a study from Italy, 11 of 201 patients (5.4 %) who regained weight after laparoscopic sleeve gastrectomy underwent laparoscopic re-sleeve gastrectomy with a significant decrease in mean BMI and increase in mean percentage of EWL at 1 year follow-up [47]. Rebibo et al. compared 15 patients who underwent re-sleeve gastrectomy to 30 patients who underwent primary sleeve gastrectomy, and the leak rate for the former group was 13 % (2/15), with less weight loss [51]. Dapri et al. reported a leak in one of seven patients who underwent re-sleeve gastrectomy [52]. However, two series of patients who underwent re-sleeve gastrectomy report no post-procedural leaks [53, 54]. This approach is typically reserved for patients with a dilated sleeve or fundus who refuse conversion to a bypass procedure.
In a study from Austria, 8 out of 73 patients underwent conversion procedure from a laparoscopic sleeve gastrectomy to laparoscopic Roux-en-y gastric bypass (five of the eight were for weight regain). None of these five patients were found to have significant sleeve dilatation. After conversion, a mean weight reduction of 15.2 ± 8 kg (range, 6–25 kg) was achieved within a follow-up from 1 to 52 months [55]. In a group of high risk, high BMI patients, Cottam et al. showed that a second stage RYGB can result in continued weight loss after LSG. One hundred twenty-six patients with mean BMI of 65 underwent LSG with an overall EWL of 46 % at 1 year. Thirty-six patients underwent a conversion procedure to RYGB 1 year after the LSG. That subgroup of patients had a mean BMI of 49 at the time of the conversion and this decreased to a mean BMI of 39 six months after conversion to RYGB with continued improvement in comorbidity status [56]. This study demonstrated the utility of LSG as a risk management strategy in high BMI patients. On the other hand, there will be a subset of these patients who can maintain long-term weight loss after LSG. In a long-term follow-up study of the same patient group, Eid et al. showed that 69 of those patients who did not return for the second stage bypass procedure were able to maintain 48 % EWL and good comorbidity improvement 6–8 years after LSG [57].
These studies highlight why the sleeve gastrectomy has become so popular: It is an effective primary operation but leaves the surgeon several safe and effective options for conversion for patients who do not achieve sufficient weight loss or have weight regain over time.
In a study by Carmeli et al., 19 patients underwent a conversion procedure after sleeve gastrectomy due to IWL (nine underwent duodenal switch and ten underwent gastric bypass). Duodenal switch yields a greater weight loss than gastric bypass, but both are feasible and effective conversion procedures after failed sleeve gastrectomy [58]. The two major advantages of duodenal switch as the conversion procedure are the avoidance of entrance into the area of scarred stomach, and revisability of the malabsorptive component (altering common channel length). The same group from Israel favored gastric bypass as the conversion procedure compared to duodenal switch if the patient had a high operative risk, vitamin deficiency , prior small bowel resection, improvement in diabetes and hypertension after sleeve gastrectomy, or BMI less than 50 prior to the sleeve gastrectomy [58].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







