The improved survival with sipuleucel-T, an autologous antigen-presenting cell–based agent, for the treatment of patients with metastatic asymptomatic and minimally symptomatic castration-resistant prostate cancer supports immunotherapy as a valid approach. Also, multiple novel immunotherapeutic approaches are undergoing vigorous investigation. T-lymphocyte checkpoint blockade and poxvirus-based prime-boost approaches are in phase III evaluation. Other immunotherapeutic platforms undergoing early investigation include radioimmunoconjugates and adenovirus-based, DNA-based, and Listeria-based approaches. The development of predictive markers for immune response that translate into improved long-term outcomes is important. This article reviews the emerging data and the unique strengths and weaknesses of these approaches.
- •
Sipuleucel-T is approved for the therapy of asymptomatic or minimally symptomatic men with metastatic castration-resistant prostate cancer.
- •
Immunotherapy seems to be a valid strategy and further vigorous development of other promising modalities is ongoing (eg, T-cell checkpoint inhibitor, ipilimumab, and the poxvirus-based agent, PROSTVAC-VF TRICOM).
- •
Rational development of immunotherapy needs to focus on biomarkers predictive of benefit.
- •
Immunotherapy seems to generally yield survival benefits with little evidence for early clinical benefits.
- •
Clinical trials evaluating immunotherapy in early-disease and low-disease burden settings should be vigorously supported because this strategy may yield the greatest benefits.
Introduction
Prostate cancer is well suited for developing immunotherapy owing to early detection, relatively indolent pace of progression, and the expression of organ-specific antigens in a nonessential organ. The rationale for immunotherapy, for malignancies in general, is predicated on overcoming the evasion of the immune system owing to defective tumor antigen processing and presentation by APCs to T lymphocytes, presumably the primary drivers of the host antitumor immune response. The defective presentation may be partly due to downregulation of major histocompatibility complex (MHC) class I molecules. Malignancies also promote an immunosuppressive microenvironment resulting from immunosuppressive cytokines (interleukin [IL]-4, IL-6, IL-10, transforming growth factor-beta, vascular endothelial growth factor, tumor necrosis factor), arachidonic acid metabolites, and depletion of tryptophan (due to overexpressed indoleamine 2, 3-dioxygenase). These inhibitory pathways occur in conjunction with the increased activity of immunosuppressive cells. These include tolerogenic APCs, myeloid-derived suppressive cells, T REG cells, T-cell receptor (TCR) dysfunction, and upregulation of checkpoint pathways that inhibit T-cell response (eg, cytotoxic T lymphocyte-associated antigen 4 [CTLA-4] and programmed cell death 1 [PD-1]). Thus, enhanced presentation of the tumor antigen to the immune system and inhibiting pathways that suppress the immune response may both be anticipated to confer antitumor activity.
The success of sipuleucel-T (Provenge, APC8015, Dendreon Corp, WA, USA) in men with metastatic castration-resistant prostate cancer (CRPC) provides the impetus to further develop immunotherapy to treat this disease ( Table 1 ). Multiple novel immunotherapeutic platforms are emerging. In addition to novel APC-based agents, promising immunotherapeutic approaches are undergoing vigorous investigation. These include T-lymphocyte checkpoint blockade, poxvirus-based immunotherapy, radioimmunoconjugates (RICs), as well as designer T lymphocytes, allogeneic-cell line-based, adenovirus-based, peptide-based, DNA-based, and Listeria -based approaches ( Fig. 1 ). This article reviews current immunotherapy with sipuleucel-T and some of the most promising emerging immunotherapeutic approaches for CRPC.
| Trial (First Author) | Target Antigen | Phase of Trial | N | Standard Regimen | Experimental Regimen | Clinical Outcomes |
|---|---|---|---|---|---|---|
| Kantoff et al, 2010 | PAP | III | 512 | Autologous APCs not pulsed with antigen | Autologous APCs pulsed with PAP-GM-CSF fusion protein (sipuleucel-T) | Extended median OS (25.8 vs 21.7 mo, HR = 0.77; P = .02) and 3-y OS (31.7% vs 23.0%) |
| Small et al, 2006 | PAP | II | 127 | Autologous APCs not pulsed with antigen | Autologous APCs pulsed with PAP-GM-CSF fusion protein (sipuleucel-T) | Extended median OS (25.8 vs 21.4 mo; P = .01 |
| Kantoff et al, 2010 | PSA | II | 125 | Empty poxvirus vector + placebo | PROSTVAC-TRICOM + GM-CSF | Extended median OS (25.1 vs 16.6 mo, P = .006) and 3-y OS (30% vs 17%) |
| Kaufman et al, 2004 | PSA | II | 64 | PROSTVAC-TRICOM rF-PSA × 4 | PROSTVAC-TRICOM rF-PSA × 3 → rV-PSA or rV-PSA × 1→ rF-PSA × 3 | Trend for PSA progression favoring priming dose of rV-PSA. |
| Gulley et al, 2010 | PSA | II | 32 | PROSTVAC-TRICOM | PROSTVAC-TRICOM + GM-CSF (multiple doses) | 13 of 28 evaluable subjects had >twofold increases in PSA-specific T-cell responses by ELISPOT; 4 of 5 high responders survived >40 mo and others had a median OS of 20 mo |
| Arlen et al, 2006 | PSA | II | 28 | PROSTVAC-TRICOM→ Docetaxel-dexamethasone | PROSTVAC-TRICOM Plus docetaxel-dexamethasone | Median PFS on docetaxel was 6.1 mo after receiving vaccine vs 3.7 mo in historical controls |
| Noguchi et al, 2010 | PPV a | II | 57 | EMP | EMP plus PPV | PPV plus EMP was associated with improvement in PSA-PFS compared with EMP (8.5 vs 2.8 mo) |
| Higano et al, 2009 | Multiple prostate antigens | III | 626 | Docetaxel-prednisone | GVAX | <30% chance of improvement in OS led to early termination |
| Small et al, 2009 | Multiple prostate antigens | III | 408 | Docetaxel-prednisone | Docetaxel plus GVAX | Shorter median OS (12.2 vs 14.1 mo, P = .0076) for GVAX-docetaxel |

Autologous APC-based immunotherapy
Sipuleucel-T
Sipuleucel-T was designed for targeted induction of the immune system to recognize and specifically eradicate prostate tumor cells. In the laboratory, the APCs are exposed to a prostate antigen-containing fusion protein followed by infusion, which is predicated to train the immune system to generate prostate-specific T cells. Sipuleucel-T is based on a platform of CD54+ APCs collected by leukapheresis and pulsed with PA2024, which is a prostatic acid phosphatase (PAP)–granulocyte-macrophage colony-stimulating factor (GM-CSF) fusion construct. A consistent extension in survival in two smaller randomized trials totaling 225 men (n = 127 and n = 98, respectively), followed by the pivotal immunotherapy for prostate adenocarcinoma treatment (IMPACT) phase III trial (n = 512). The IMPACT trial included men with asymptomatic or minimally symptomatic metastatic CRPC who were randomized in a 2:1 ratio to sipuleucel-T or APCs not pulsed with PA2024. Men with visceral metastasis and fewer than 3 months beyond chemotherapy were excluded. Approximately 85% of subjects were chemotherapy naïve. Subjects underwent three leukapheresis, 2 weeks apart, followed 2 to 3 days later by an infusion of sipuleucel-T or placebo. Each dose of sipuleucel-T contained greater than or equal to 40 million large cells expressing CD54 administered intravenously (IV) over 60 minutes following premedication with acetaminophen and an antihistamine.
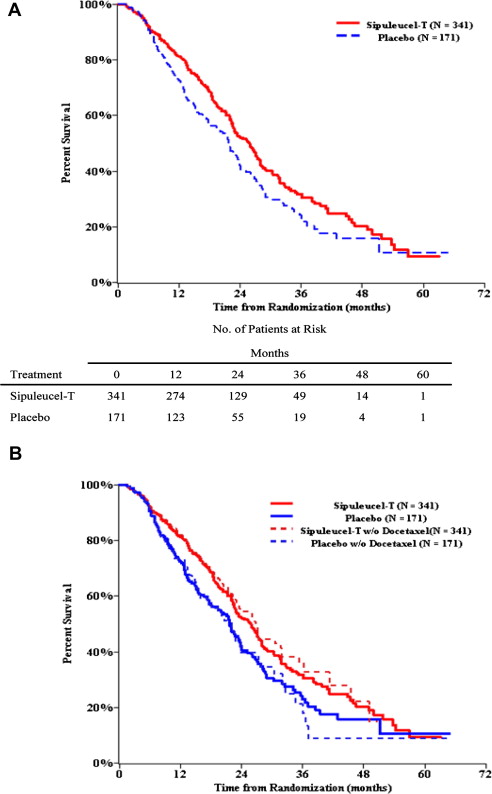
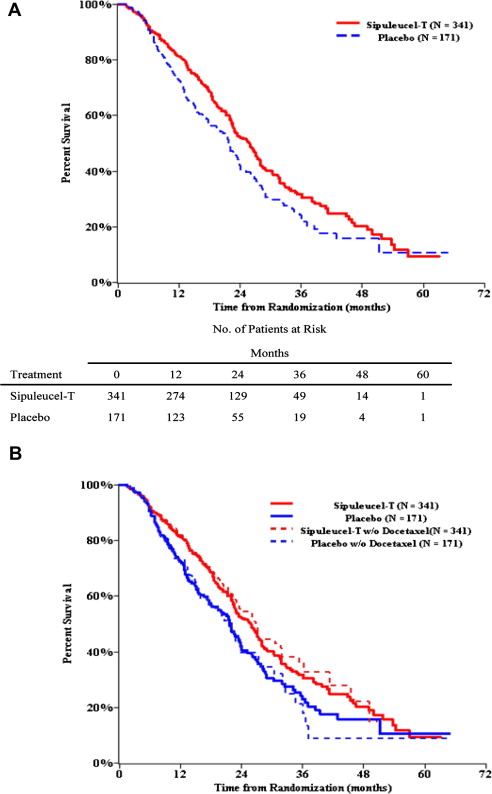
The IMPACT trial demonstrated an extension of median survival (25.8 vs 21.7 months, hazard ratio [HR] = 0.77; P = .02) and 3-year survival (31.7% vs 23.0%), with the survival curves exhibiting delayed separation at approximately 6 months ( Fig. 2 ). However, few prostate-specific antigen (PSA) declines or objective responses occurred and no delay was seen in time to first progression. A subsequent retrospective analysis integrating the results of IMPACT and the earlier, smaller trials detected delayed separation in the time to disease-related pain at approximately 6 months, such that 39.3% of sipuleucel-T versus 18.9% of controls were pain-free at 12 months (HR = 0.84 [95% CI: 0.64, 1.12]; P = .24). There was no preferential benefit based on baseline PSA, lactate dehydrogenase, alkaline phosphatase, number of bone metastases, Gleason score, performance status, and pain. A similar proportion of men (∼55%) in both groups received docetaxel at a median of 12 to 13 months after study therapy, but sensitivity analyses suggested that docetaxel use was not responsible for differences in outcomes. The survival effect was observed despite crossover of 49.1% placebo subjects to initial salvage frozen and stored sipuleucel-T, and at any time, in 63.7% of subjects. Intriguingly, crossover subjects (n = 109) enjoyed an increment in postprogression survival relative to untreated controls (n = 62) with a median survival of 23.8 versus 11.6 months (HR = 0.52 [95% CI: 0.37, 0.73]; P = .0001). Because such an analysis may be confounded by more favorable subjects who are crossing over from placebo to sipuleucel-T, an additional analysis was undertaken by invoking the rank-preserving structural failure time model. This analysis also suggested an extension of survival in the crossover subjects.
The toxicity profile was excellent with manageable infusional adverse events including fever (22.5%) and chills (51.2%). The ongoing PROCEED registry study (NCT01306890) is collecting toxicity data to allay concerns regarding a possible non–statistically significant increase in cerebral hemorrhage seen in initial studies. Concerns regarding cost remain, although the Centers for Medicare and Medicaid Services has deemed sipuleucel-T as reasonable and necessary.
Anti-PA2024 antibody titer greater than 400 was noted in 66.2% of subjects in the sipuleucel-T group and 2.9% in the placebo group and was provocatively associated with survival benefit ( P <.001). Antibody responses against PAP (28.5% vs 1.4%) and T-cell proliferation responses to both PA2024 (73% vs 12.1%) and PAP (27.3% vs 8%) were more frequent with sipuleucel-T but were not statistically significantly associated with survival. Whereas cytotoxic T-cell upregulation is thought to be more critical for antitumor activity, the association of the antibody response with outcomes may merely be a surrogate for better T-cell responses or may reflect imprecise assays to measure T-cell responses. The quality of the vaccine product may predict benefit as suggested by the association of CD54 upregulation and nucleated cell counts with survival in placebo subjects who crossed over to frozen product. The ProAct trial is evaluating the differences in immune response by sipuleucel-T manufactured with different concentrations of PA2024. Another open-label phase II trial (OpenAct) extensively evaluates cellular and humoral immune system activation.
Sipuleucel-T has also demonstrated activity in earlier stages of prostate cancer. PROTECT (PROvenge Treatment and Early Cancer Treatment) was a randomized double-blind phase III trial (n = 176) that investigated sipuleucel-T after a 3- to 4-month course of androgen deprivation therapy (ADT) in biochemically recurrent nonmetastatic castration-sensitive disease. Sipuleucel-T subjects displayed a 48% increase in PSA-doubling time (DT) following testosterone recovery (155 vs 105 days, P = .038). However, clinical outcomes were not statistically different. Remarkably, T-cell proliferative and enzyme-linked immunosorbent spot (ELISPOT) responses to PA2024 were observed at a median of 22.6 months and up to 67.3 months. In another trial, sipuleucel-T favorably modified PSA kinetics and produced immune responses when combined with bevacizumab in hormone-naïve prostate cancer. A recent report demonstrated the biologic activity of neoadjuvant sipuleucel-T preceding radical prostatectomy. Greater than twofold increases in CD3 + and CD4 + T cells were observed at the tumor rim where benign and malignant glands interface, compared with the pretreatment biopsy.
An ongoing phase III international trial (P-10-4) is investigating the efficacy of combining ADT with sipuleucel-T in metastatic castration-sensitive prostate cancer ( Table 2 ). Other randomized phase II trials use immune response–based primary endpoints to evaluate the sequencing of sipuleucel-T and hormonal agents (see Table 2 ). In one study, sipuleucel-T is administered 2 weeks before or 12 weeks into a 12-month course of ADT for hormone-sensitive metastatic disease. Another trial randomizes men with metastatic CRPC to receive sipuleucel-T followed by abiraterone acetate (in combination with prednisone), or to concurrent therapy with both of these agents.
| Molecular Target | Institution | Trial Identifier | Phase of Trial | Class of Therapeutic Agent | Line of Therapy | Standard Arm | Experimental Arm |
|---|---|---|---|---|---|---|---|
| CTLA-4 | International | NCT01057810 | III | MAb | First, metastatic CRPC | XRT → Placebo | XRT→ Ipilimumab |
| CTLA-4 | International | NCT00861614 | III | MAb | Second, metastatic CRPC | XRT → Placebo | XRT→ Ipilimumab |
| PAP | International | P-10–4 | III | Autologous APC | Castration-sensitive, metastatic | ADT | ADT + Sipuleucel-T |
| PAP | Multicenter | NCT01431391 | II | Autologous APC | Castration-sensitive, metastatic | ADT + Sipuleucel-T | ADT → Sipuleucel-T |
| PAP | Multicenter | NCT01487863 | II | Autologous APC | Metastatic CRPC | Sipuleucel-T → AA+ P | Sipuleucel-T + AA+ P |
| PSMA | Cornell-led multicenter | NCT00859781 | II | Radioisotope Lu-177 tagged MAb | Nonmetastatic CRPC, phase II | KC + HC | KC + HC + Lu-177-J591 |
| PSA | Multicenter | NCT01322490 | III | Virus-based antigen and costimulatory molecule expression | First, metastatic CRPC, phase III | Placebo | PROSTVAC-TRICOM |
| PSA | NIH, USA | NCT00450463 | II | Virus-based antigen and costimulatory molecule expression | First, nonmetastatic CRPC, phase II | Flutamide | Flutamide + PROSTVAC-TRICOM |
| PSA | University of Iowa | NCT00583752 | II | Adenovirus-based | Nonmetastatic, hormone naïve | Adenovirus-PSA vaccine→ADT | Adenovirus-PSA vaccine + ADT |
| PAP | University of Wisconsin | NCT01341652 | II | Plasmid DNA | Nonmetastatic, hormone naïve | GM-CSF | DNA vaccine + GM-CSF |
| PAP | University of Wisconsin | NCT00849121 | II | Plasmid DNA | Nonmetastatic CRPC | DNA vaccine Induction→maintenance | DNA vaccine → tailored maintenance based on immune response |
| Gene-mediated immunotherapy | Multicenter | NCT01436968 | III | AdV-HSV-tk + valacyclovir | Localized high-risk untreated | Radiation | Radiation → Vaccine |
Emerging Autologous APC-Based Agents
APCs engineered to express drug-inducible D40 receptors using an adenovirus vector were preclinically shown to engage CD4+ T-helper cells within the lymph node paracortex to augment cytotoxic lymphocyte activation. The CD40 receptor was then activated in vivo by a dimerizer (AP1903) in a temporally controlled manner, which was expected to prolong the activated state of APCs. A phase I-II clinical trial enrolled 18 men with metastatic CRPC and reported feasibility and promising activity for this approach when targeting prostate-specific membrane antigen (PSMA).
Autologous APC-based immunotherapy
Sipuleucel-T
Sipuleucel-T was designed for targeted induction of the immune system to recognize and specifically eradicate prostate tumor cells. In the laboratory, the APCs are exposed to a prostate antigen-containing fusion protein followed by infusion, which is predicated to train the immune system to generate prostate-specific T cells. Sipuleucel-T is based on a platform of CD54+ APCs collected by leukapheresis and pulsed with PA2024, which is a prostatic acid phosphatase (PAP)–granulocyte-macrophage colony-stimulating factor (GM-CSF) fusion construct. A consistent extension in survival in two smaller randomized trials totaling 225 men (n = 127 and n = 98, respectively), followed by the pivotal immunotherapy for prostate adenocarcinoma treatment (IMPACT) phase III trial (n = 512). The IMPACT trial included men with asymptomatic or minimally symptomatic metastatic CRPC who were randomized in a 2:1 ratio to sipuleucel-T or APCs not pulsed with PA2024. Men with visceral metastasis and fewer than 3 months beyond chemotherapy were excluded. Approximately 85% of subjects were chemotherapy naïve. Subjects underwent three leukapheresis, 2 weeks apart, followed 2 to 3 days later by an infusion of sipuleucel-T or placebo. Each dose of sipuleucel-T contained greater than or equal to 40 million large cells expressing CD54 administered intravenously (IV) over 60 minutes following premedication with acetaminophen and an antihistamine.
The IMPACT trial demonstrated an extension of median survival (25.8 vs 21.7 months, hazard ratio [HR] = 0.77; P = .02) and 3-year survival (31.7% vs 23.0%), with the survival curves exhibiting delayed separation at approximately 6 months ( Fig. 2 ). However, few prostate-specific antigen (PSA) declines or objective responses occurred and no delay was seen in time to first progression. A subsequent retrospective analysis integrating the results of IMPACT and the earlier, smaller trials detected delayed separation in the time to disease-related pain at approximately 6 months, such that 39.3% of sipuleucel-T versus 18.9% of controls were pain-free at 12 months (HR = 0.84 [95% CI: 0.64, 1.12]; P = .24). There was no preferential benefit based on baseline PSA, lactate dehydrogenase, alkaline phosphatase, number of bone metastases, Gleason score, performance status, and pain. A similar proportion of men (∼55%) in both groups received docetaxel at a median of 12 to 13 months after study therapy, but sensitivity analyses suggested that docetaxel use was not responsible for differences in outcomes. The survival effect was observed despite crossover of 49.1% placebo subjects to initial salvage frozen and stored sipuleucel-T, and at any time, in 63.7% of subjects. Intriguingly, crossover subjects (n = 109) enjoyed an increment in postprogression survival relative to untreated controls (n = 62) with a median survival of 23.8 versus 11.6 months (HR = 0.52 [95% CI: 0.37, 0.73]; P = .0001). Because such an analysis may be confounded by more favorable subjects who are crossing over from placebo to sipuleucel-T, an additional analysis was undertaken by invoking the rank-preserving structural failure time model. This analysis also suggested an extension of survival in the crossover subjects.
The toxicity profile was excellent with manageable infusional adverse events including fever (22.5%) and chills (51.2%). The ongoing PROCEED registry study (NCT01306890) is collecting toxicity data to allay concerns regarding a possible non–statistically significant increase in cerebral hemorrhage seen in initial studies. Concerns regarding cost remain, although the Centers for Medicare and Medicaid Services has deemed sipuleucel-T as reasonable and necessary.
Anti-PA2024 antibody titer greater than 400 was noted in 66.2% of subjects in the sipuleucel-T group and 2.9% in the placebo group and was provocatively associated with survival benefit ( P <.001). Antibody responses against PAP (28.5% vs 1.4%) and T-cell proliferation responses to both PA2024 (73% vs 12.1%) and PAP (27.3% vs 8%) were more frequent with sipuleucel-T but were not statistically significantly associated with survival. Whereas cytotoxic T-cell upregulation is thought to be more critical for antitumor activity, the association of the antibody response with outcomes may merely be a surrogate for better T-cell responses or may reflect imprecise assays to measure T-cell responses. The quality of the vaccine product may predict benefit as suggested by the association of CD54 upregulation and nucleated cell counts with survival in placebo subjects who crossed over to frozen product. The ProAct trial is evaluating the differences in immune response by sipuleucel-T manufactured with different concentrations of PA2024. Another open-label phase II trial (OpenAct) extensively evaluates cellular and humoral immune system activation.
Sipuleucel-T has also demonstrated activity in earlier stages of prostate cancer. PROTECT (PROvenge Treatment and Early Cancer Treatment) was a randomized double-blind phase III trial (n = 176) that investigated sipuleucel-T after a 3- to 4-month course of androgen deprivation therapy (ADT) in biochemically recurrent nonmetastatic castration-sensitive disease. Sipuleucel-T subjects displayed a 48% increase in PSA-doubling time (DT) following testosterone recovery (155 vs 105 days, P = .038). However, clinical outcomes were not statistically different. Remarkably, T-cell proliferative and enzyme-linked immunosorbent spot (ELISPOT) responses to PA2024 were observed at a median of 22.6 months and up to 67.3 months. In another trial, sipuleucel-T favorably modified PSA kinetics and produced immune responses when combined with bevacizumab in hormone-naïve prostate cancer. A recent report demonstrated the biologic activity of neoadjuvant sipuleucel-T preceding radical prostatectomy. Greater than twofold increases in CD3 + and CD4 + T cells were observed at the tumor rim where benign and malignant glands interface, compared with the pretreatment biopsy.
An ongoing phase III international trial (P-10-4) is investigating the efficacy of combining ADT with sipuleucel-T in metastatic castration-sensitive prostate cancer ( Table 2 ). Other randomized phase II trials use immune response–based primary endpoints to evaluate the sequencing of sipuleucel-T and hormonal agents (see Table 2 ). In one study, sipuleucel-T is administered 2 weeks before or 12 weeks into a 12-month course of ADT for hormone-sensitive metastatic disease. Another trial randomizes men with metastatic CRPC to receive sipuleucel-T followed by abiraterone acetate (in combination with prednisone), or to concurrent therapy with both of these agents.
| Molecular Target | Institution | Trial Identifier | Phase of Trial | Class of Therapeutic Agent | Line of Therapy | Standard Arm | Experimental Arm |
|---|---|---|---|---|---|---|---|
| CTLA-4 | International | NCT01057810 | III | MAb | First, metastatic CRPC | XRT → Placebo | XRT→ Ipilimumab |
| CTLA-4 | International | NCT00861614 | III | MAb | Second, metastatic CRPC | XRT → Placebo | XRT→ Ipilimumab |
| PAP | International | P-10–4 | III | Autologous APC | Castration-sensitive, metastatic | ADT | ADT + Sipuleucel-T |
| PAP | Multicenter | NCT01431391 | II | Autologous APC | Castration-sensitive, metastatic | ADT + Sipuleucel-T | ADT → Sipuleucel-T |
| PAP | Multicenter | NCT01487863 | II | Autologous APC | Metastatic CRPC | Sipuleucel-T → AA+ P | Sipuleucel-T + AA+ P |
| PSMA | Cornell-led multicenter | NCT00859781 | II | Radioisotope Lu-177 tagged MAb | Nonmetastatic CRPC, phase II | KC + HC | KC + HC + Lu-177-J591 |
| PSA | Multicenter | NCT01322490 | III | Virus-based antigen and costimulatory molecule expression | First, metastatic CRPC, phase III | Placebo | PROSTVAC-TRICOM |
| PSA | NIH, USA | NCT00450463 | II | Virus-based antigen and costimulatory molecule expression | First, nonmetastatic CRPC, phase II | Flutamide | Flutamide + PROSTVAC-TRICOM |
| PSA | University of Iowa | NCT00583752 | II | Adenovirus-based | Nonmetastatic, hormone naïve | Adenovirus-PSA vaccine→ADT | Adenovirus-PSA vaccine + ADT |
| PAP | University of Wisconsin | NCT01341652 | II | Plasmid DNA | Nonmetastatic, hormone naïve | GM-CSF | DNA vaccine + GM-CSF |
| PAP | University of Wisconsin | NCT00849121 | II | Plasmid DNA | Nonmetastatic CRPC | DNA vaccine Induction→maintenance | DNA vaccine → tailored maintenance based on immune response |
| Gene-mediated immunotherapy | Multicenter | NCT01436968 | III | AdV-HSV-tk + valacyclovir | Localized high-risk untreated | Radiation | Radiation → Vaccine |
Emerging Autologous APC-Based Agents
APCs engineered to express drug-inducible D40 receptors using an adenovirus vector were preclinically shown to engage CD4+ T-helper cells within the lymph node paracortex to augment cytotoxic lymphocyte activation. The CD40 receptor was then activated in vivo by a dimerizer (AP1903) in a temporally controlled manner, which was expected to prolong the activated state of APCs. A phase I-II clinical trial enrolled 18 men with metastatic CRPC and reported feasibility and promising activity for this approach when targeting prostate-specific membrane antigen (PSMA).
Poxvirus-based immunotherapy
Viral vaccines display high immunogenicity, do not require patient-specific manufacture, and are capable of carrying a large payload of genetic material. Poxviruses engineered to carry a tumor antigen can infect host cells and replicate within the cytoplasm. The tumor antigen may then generate neutralizing antibodies to the encoded antigen (eg, PSA) and may also be taken up by APCs and presented to T cells. Thus, in addition to an immune response against the poxvirus, a tumor antigen-specific immune response may be anticipated. The critical barrier is the induction of antibodies against the poxvirus-based vector, which may neutralize the immunogenicity of a booster dose. To overcome this problem, a different poxvirus or avipoxvirus vector has been used as the booster. The poxvirus agent in trials, PROSTVAC-VF TRICOM (Bavarian Nordic, Washington, DC, USA), consists of an initial priming dose of recombinant vaccinia (rV) followed by a booster dose of recombinant fowlpox (rF) subcutaneously. Each of these vectors was engineered to express PSA and TRICOM consisting of the costimulatory molecules intercellular adhesion molecule 1 (ICAM-1 [CD54]), B7.1 (CD80), and leukocyte function-associated antigen 3 (CD58).
An initial randomized phase II trial evaluated different schedules of this prime-boost approach. This trial randomized 64 subjects with PSA progression after local therapy to receive rF-PSA × 4, rF-PSA × 3 → rV-PSA, or rV-PSA × 1→ rF-PSA × 3. Among eligible subjects, 45.3% were free of PSA progression at 19.1 months and 78.1% demonstrated clinical progression-free survival (PFS). There was a trend for PSA progression favoring the group that received a priming dose of rV-PSA. Although no significant increases in anti-PSA antibody titers were detected, 46% of subjects demonstrated an increase in PSA-reactive T cells.
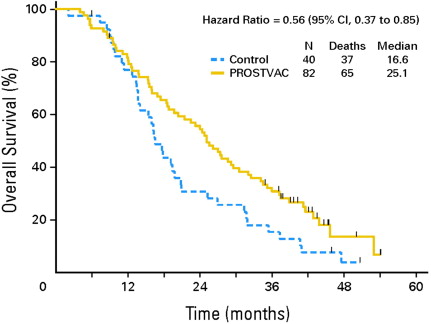
Subsequently, a double-blind randomized phase II trial enrolled 122 subjects with chemotherapy-naïve, minimally symptomatic metastatic CRPC, Gleason score less than or equal to 7 and no visceral metastasis (see Table 1 ). Therapy was administered subcutaneously on days 1, 14, 28, 56, 84, 112, and 140 and consisted of one priming dose and 6 booster doses. The PROSTVAC arm subjects received priming with rV-PSA-TRICOM followed by boosts using rF-PSA-TRICOM. GM-CSF was administered within 5 mm of vaccination as an adjuvant on the day of and for 3 consecutive days thereafter. The control arm received priming immunization with empty vaccinia vector and boosts with empty fowlpox vector, in conjunction with placebo saline injections instead of GM-CSF. Nineteen of the 40 subjects in the control arm, crossed over to PROSTVAC-VF. PFS was similar in the two groups ( P = .56). However, with mature follow-up, PROSTVAC-VF TRICOM conferred a significant extension of median survival (25.1 vs 16.6 months, P = .006) and 3-year survival (30% vs 17%), again characterized by a delayed separation in survival curves ( Fig. 3 ).