 ALTERED IMMUNITY IN CHRONIC KIDNEY DISEASE
ALTERED IMMUNITY IN CHRONIC KIDNEY DISEASE
The immune system is a complex orchestration of cells, cytokines, and other molecules that act in a paracrine, autocrine, or endocrine manners to protect the human organism against disease. Uremia is known to be associated with a state of immune dysfunction characterized by both immune depression—that likely contributes to increased incidence and severity of microbial infections and impaired response to vaccination—and immune activation that results in a persistent inflammatory state that might contribute to PEW, atherosclerosis, CVD, and high mortality observed in this group of patients (14).
Within the complex immune system, the two major branches, innate and acquired immunity, are altered in uremic patients. The possible causes responsible for these disturbances in the uremic milieu are linked to the decline of GFR and the noxious effect of uremic toxins that accumulate due to kidney disease; however, numerous other factors such as comorbidities, other superimposed illnesses, genetic predisposition, and therapeutic interventions including the dialysis procedure, to name a few, play important roles in the pathophysiology of these immune disturbances.
With regard to the immune disorders observed in CKD, disturbances in the number and especially the function of basically all immune cells have been described. In general, the functional alterations observed include spontaneous activation, decreased phagocytic capacity, and increased apoptosis, leading to increased generation of cytokines and reactive oxygen species (ROS) and diminished defensive capacity (15). The different immune cells abnormalities observed in uremic patients are summarized in TABLE 20.1.
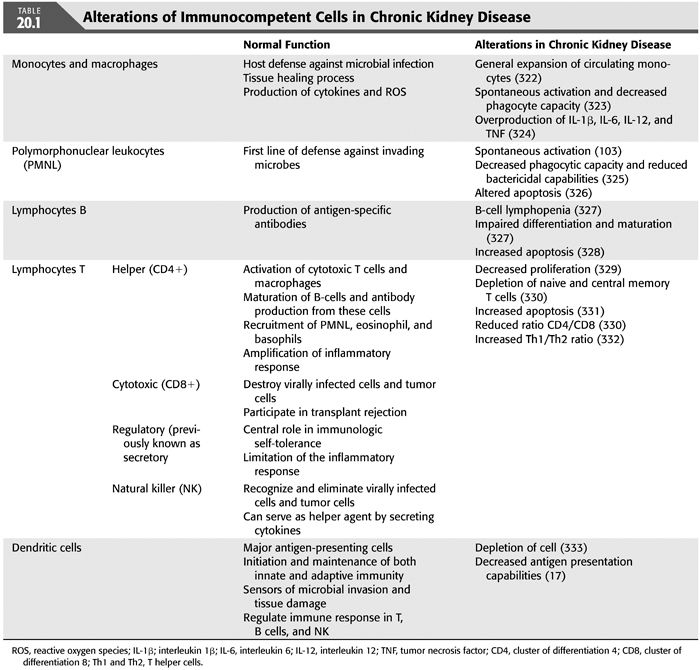
Among the mechanisms responsible for the compromised function of the immune cells, those concerning the pattern-recognition receptors, especially the Toll-like receptors (TLR), deserve a special mention. Pattern-recognition receptors play a role in innate immunity by recognizing pathogens through specific pathogen-associated molecular patterns (PAMPs) (16) and triggering effector cells to perform their functions. In uremic patients, disorders in the pattern-recognition receptors system result in impaired function of cells engaged in innate immunity, leading among others, to decreased endocytosis and impaired maturation (17). In the uremic milieu, some of these receptors have been shown to be upregulated, while others are downregulated, leading to different consequences. For instance, upregulation of mannose-binding lecithin (a type of pattern-recognition receptor) is associated with worse patient and graft survival after simultaneous pancreas-kidney transplantation (18), while low levels associated with increased mortality in infected hemodialysis (HD) patients (19). Toll-like receptors belong also to the family of signaling pattern-recognition receptors that is involved in the maturation of dendritic cells, phagocytic functions, and activation of the complement pathway and production of numerous cytokines, such as interleukin 1β (IL-1β), interleukin 6 (IL-6), and TNF (20). Disorders in this group of receptors are responsible for impaired function of antigen-presenting cells (APCs), leading to more severe illness and increased mortality in case of invading pathogens diseases (14). Interestingly, a study by Kiechl et al. (21) reported that, although subjects with TLR4 polymorphism, which led to TLR4 downregulation, were found to be more susceptible to severe bacterial infections; they had a lower risk of carotid atherosclerosis and reduced intima-media thickness. These results suggest that impaired innate immunity might protect against atherosclerosis, thus resulting in lower cardiovascular mortality because of attenuated receptor signaling and diminished inflammatory response. If a similar situation is present in end-stage kidney disease (ESKD) patients, ESKD patients with impairment of immunity may have a survival advantage in the form of lower risk of CVD. But, again, this alteration may be linked to increased risk of infections and thereby inflammation.
Cytokine Dysregulation in Chronic Kidney Disease
As a consequence of the altered function of the immune cells, a state of hypercytokinemia is characteristic in the uremic milieu, where the delicate equilibrium between proinflammatory cytokines and their inhibitors is clearly dysregulated (22,23). Also, due to the fact that the kidneys are major sites for elimination of many of these cytokines, renal failure contributes notably to the hypercytokinemic state. Additionally, the dialysis procedure per se stimulates circulating nuclear cells, inducing cytokine production (24) and making them respond more vigorously after exposure to endotoxin (25).
To properly understand the state of cytokine dysregulation present in CKD patients, a number of considerations involving cytokine measurements should be made (26). First, most of the published studies focus on selected cytokines measured in plasma, culture supernatants, or in association with circulating cells. However, cytokines are “moving targets” counterbalanced by inhibitors or other cytokines with opposite effects. Second, cytokines rarely act alone because they stimulate a variety of cell types to produce and secrete other cytokines in a cascade fashion. Elevation of one cytokine immediately leads to up- or downregulation of several others. As many of the effects of cytokines are local, not systemic, these paracrine effects of cytokines are hard to detect. Third, pro- and anti-inflammatory cytokines bind to specific cytokine carriers (such as α2-macroglobulin) and these different binding proteins may serve as extracellular cytokine reservoirs and protective shields against degradation. Therefore, it is important to take into account that established immunoassays to detect cytokines usually are not able to distinguish between active proteins and those proteins that are blocked by their specific inhibitors. To understand this complex orchestration, selected cytokines will be reviewed with regard to our current understanding of the uremic cytokine misbalance.
Interleukin 6
Different cells, including T-lymphocytes, macrophages, monocytes, endothelial cells, adipocytes, and fibroblasts, produce IL-6. IL-6 is involved in the production of neutrophils and proliferation of B-lymphocytes as well as in the stimulation of the liver to produce acute-phase proteins. IL-6 also regulates the production of adhesion molecules and induces the secretion of monocyte chemotactic protein, an important mediator for the release of other cytokines, such as TNF and IL-1β, which subsequently amplify the inflammatory response (27). Notably, IL-6 exhibits both pro- and anti-inflammatory effects and promotes inflammatory events through the activation and proliferation of lymphocytes as well as differentiation of B cells and leukocyte recruitment.
Numerous factors in the uremic milieu may stimulate the production of IL-6, and the kidney has been suggested to play an important role in the clearance of IL-6 (28–30). Indeed, Bolton et al. (31) found in a multiple regression analysis that serum creatinine was the sole determinant of plasma IL-6 levels in a group of predialysis and dialysis patients. Other causes responsible for the elevated IL-6 levels in CKD include comorbidities, persistent infections, such as Chlamydia pneumoniae (32), fluid overload, sympathetic overactivation, and oxidative stress, conditions that often appear as renal function declines (33).
Elevated concentration of circulating IL-6 is strongly associated with comorbidity and is a powerful predictor of CVD and all-cause mortality in dialysis patients (34,35). This prognostic value might be related to some independent proatherogenic properties showed by IL-6 in the early stages of atherosclerosis (36), in contrast to the putative antiatherogenic effects of CRP (37). Increased expression of IL-6 has been observed at the fibrous plaque stage of the atherosclerotic process (38), and elevated IL-6 levels have been linked to the progression of carotid atherosclerosis in patients with CKD stage 5 (32). The pathways by which IL-6 could contribute to the development of atherosclerosis include diverse metabolic, endothelial, and coagulant mechanisms (39).
Tumor Necrosis Factor
TNF is a pleiotropic inflammatory cytokine that has a pivotal role in regulating both pro- and anti-inflammatory mediators. TNF is an acute-phase protein that initiates the cytokine cascade and thereby increases vascular permeability. TNF is produced mainly by monocytes, macrophages, dendritic cells, and Th1 cells and is in charge of recruiting macrophages and neutrophils to the site of infection. This cytokine holds both growth-stimulating and growth inhibitory properties, and it appears to have self-regulatory properties as well. Deterioration of renal function may be one of the most important factors associated with a significant increase in TNF activity (28,40). TNF is highly multifunctional, affecting insulin resistance (41), coagulation cascade, lipid metabolism, and endothelial dysfunction (42). TNF is also associated with increased catabolism and may promote both atherosclerosis and PEW, and, consequently, higher levels of TNF associate with poor outcomes in patients with CKD (29).
Interleukin 10
Interleukin 10 (IL-10) is an essential anti-inflammatory cytokine produced mainly by immunoactive cells, such as monocytes and lymphocytes, and has been regarded as one of the most important anti-inflammatory immune-regulating cytokines. In fact, IL-10 not only downregulates proinflammatory cytokines, such as IL-1, IL-6, and TNF, but also reduces the production of chemotactic factors, such as IL-8 or CC chemokines, which may attract leukocytes to the location of inflammatory activity (43). Apart from the inhibition of proinflammatory cytokines, several other properties like antiatherogenic and antithrombotic effects have been related to IL-10 (26). IL-10 is capable of preventing the attachment of circulating immune cells to the endothelium (44) by inhibiting the secretion of chemotactic proteins from macrophages; signaling further recruitment of leukocytes to the subendothelial location of inflammation (43); and reducing the production of matrix metalloproteinases and superoxide anions (45). Additionally, it has been suggested that IL-10 prevent destabilization of the atherogenic plaque (46). As IL-10 is mainly cleared through the kidneys, its half-life is markedly increased and its plasma levels commonly elevated in CKD (47). Furthermore, uremic monocytes produce higher amounts of this cytokine compared to those of healthy individuals (48), probably as a consequence of chronic monocyte activation in patients with uremia. In patients with CKD, the most relevant production of IL-10 seems to occur in monocytes and macrophages, in response to endotoxins and activated complement fragments, agents known to mediate bioincompatibility reactions during renal replacement therapy (RRT). Therefore, dialysis treatment may contribute to the overall level of this long-acting cytokine. Despite the positive features assigned to IL-10, previous studies in CKD have failed to show an association between IL-10 and better outcomes. In fact, higher serum IL-10 levels have also been associated with increased risk of cardiovascular events during follow-up (49), results that can be explain as a consequence of the global proinflammatory uremic milieu that as a compensatory sequel also involves increased release of IL-10.
 ENHANCED SUSCEPTIBILITY TO BACTERIAL INFECTIONS IN UREMIA
ENHANCED SUSCEPTIBILITY TO BACTERIAL INFECTIONS IN UREMIA
As mentioned above, CKD is a condition associated with a state of immune dysfunction affecting both innate and acquired immunity and therefore leading to increased susceptibility to infections. Indeed, the incidence of bacterial infections is 50-fold higher in dialysis patients in comparison to the general population (32,50,51). Aside from being a consequence of altered immunity, the high infectious morbidity observed in CKD is multifactorial and the main factors involved are summarized in TABLE 20.2. The occurrence of bacterial infections in CKD is commonly related to the dialysis procedure and to the vascular access [especially central dialysis catheters (52)] that represents a continuous portal of entry for pathogens. Additionally, respiratory and urinary tract infections have a high prevalence in CKD patients. Likewise, comorbidity, especially diabetes and peripheral vascular pathology, have an important role in the incidence and prevalence of foot or leg ulcers infection commonly seen in this group of patient.

Among the septicemia episodes in CKD patients, around 40% to 70% of them are caused by gram-positive microorganisms (53–55), being Staphylococcus aureus the most commonly encountered gram-positive agent and mainly associated with vascular access infection (54). The high prevalence of S. aureus asymptomatic carriers in nose, throat, and skin, and the required repetitive punctures of the vascular access (55–57) represent important risk factors. In peritoneal dialysis (PD) patients, formation of a bacterial biofilm on the walls of the peritoneal catheter is also a frequent cause of S. aureus peritonitis (58). Interestingly, aspirin treatment was recently associated with decreased rate of catheter-associated S. aureus bacteremia in HD patients (59). On the other hand, gram-negative bacteria infections are present in approximately 25% of the episodes of bacteremia of CKD patients, and are linked to vascular access infections (53) and gastrointestinal and genitourinary tract infections (57,60), where Escherichia coli is the most common gram-negative pathogen isolated (54,55). Moreover, there is a link between the intracellular gram-negative pathogen C. pneumoniae and atherosclerotic complications in dialysis patients (51). Although this causative link remains to be demonstrated, ongoing trials using macrolide therapy will allow assessment of whether eradication of this type of infection could result in reduced atherosclerotic morbidity (61).
Furthermore, among other infectious diseases observed in CKD patients, tuberculosis deserves to be mentioned. Due to their particular immune characteristics, patients with CKD are at increased risk of development and reactivation of latent tuberculosis and exhibit a much higher prevalence than the general population (62,63). The presentation of the disease is frequently atypical, with accelerated wasting being one possible consequence of Mycobacterium tuberculosis infection. Indeed, in ancient times, tuberculosis was referred to as “phthisis,” which means “consumption.” The diagnosis of tuberculosis infection is specially challenging in CKD due to its frequent extrapulmonary location (64,65) and the scarce sensitivity of tuberculin skin test in dialysis patients, who show anergy in more than 50% of the patients (66). In the last years, new techniques like blood tests determination using stimulation of gamma interferon have been developed with better results (67); however, sometimes, a biopsy of extrapulmonary tissue is required.
Moreover, hidden infectious process, like periodontitis (68) and bowel bacterial overgrowth (69), are prevalent conditions that are usually overlooked, although they are related to deleterious outcomes. In any case, independent of the type of infection, even asymptomatic infections contribute considerably to the persistent inflammatory state observed in CKD patients and result in increased risk of morbidity and mortality (70). Emphasis on the importance of good dialysis care and implementation of routines to avoid infection in this group of susceptible patients are of fundamental importance.
 LOW-GRADE PERSISTENT INFLAMMATORY STATE
LOW-GRADE PERSISTENT INFLAMMATORY STATE
Systemic inflammation is a common feature in the uremic phenotype (7–12) and is linked to poor outcomes (71). While immune system dysfunction associated to CKD leads to a persistent inflammatory state (72,73) partially due to impaired renal elimination of proinflammatory cytokines and partially due to increased generation of cytokines (29), other factors related to dialysis technique and lifestyle are also important contributors. Moreover, it has recently become apparent that stress-induced premature senescence contributes significantly to the chronic inflammatory state of advanced CKD (74). If persistent, injurious triggers by oxidative stress, hyperphosphatemia, and uremic toxins will prevent DNA damage from being repaired, and cells will undergo senescence and secrete cytokines and growth factors (the so called senescence-associated secretory phenotype; SASP) (75).
Causes of Persistent Inflammatory State
General
A number of factors, both related and unrelated to CKD or dialysis technique, contribute to systemic inflammation in patients with uremia (FIGURE 20.2). A reduction of kidney function per se is associated with an increased inflammatory response due to the retention of circulating cytokines (26), advanced glycation end products (AGEs) (76), and pro-oxidants (33). Additional mechanisms include sympathetic overactivity (and/or blunted vagal nerve activity) and reduced production of specific cytokine inhibitors [suppressors of cytokine signaling (SOCS)]. As the cholinergic anti-inflammatory pathway is a neural mechanism that inhibits local cytokine release, blunted vagal activity may be a mechanism leading to increased inflammatory activity (77). Overhydration, a frequent complication in CKD, is also an indirect contributor to inflammation. Volume overload, via bacterial or endotoxin translocation in patients with severe gut edema, leads to immune activation and increased inflammatory cytokine production (78). Also, a strong relation between inflammation, residual renal function, and cardiac hypertrophy has been documented (79). Notwithstanding, other inflammatory diseases, such as systemic lupus erythematosus, rheumatoid arthritis and malignancies, are commonly observed in dialysis patients and can contribute to the inflammatory state, like intercurrent clinical events, that not surprisingly seem to be the most important predicting factor of elevation of CRP values in the context of uremia (80).

Causes Related to Dialysis Technique
In addition to the intrinsic inflammation accompanying CKD, patients undergoing RRT are subject to additional potential inflammation-activating factors. Factors associated with the dialysis technique itself include bioincompatible membranes and solutions, dialysate back flow, clotted access grafts, and catheter infections. Other factors, such as failed kidney transplants (81), endotoxemia (71,82), and fluid overload (83) may also influence the inflammatory process in this group of patients.
Additional Causes of Inflammation in Hemodialysis Patients
A number of factors related to HD procedure per se promote inflammation. While interestingly, dialysis-related inflammation seems to be associated with a specific genomic pattern (84); several in vivo studies imply that the membrane composition, the type and quality of dialysis, and the type of vascular access may contribute to inflammatory processes. In a randomized study, Schindler et al. (85) demonstrated that HD patients treated with polyamide membranes presented lower CRP levels compared to those exposed to cuprophane or polycarbonate membranes (see Chapter 1). In accordance, Memoli et al. (86) showed that a significant relation exists between membrane bioincompatibility and circulating levels of CRP, IL-6, and serum albumin. Likewise, it has been proposed that the amount of convective transport and the frequency of dialysis might influence in the level of inflammation (87). Short daily HD (six sessions per week of 3 hours each) was associated with a reduction in left ventricular hypertrophy and inflammatory mediators compared to conventional HD (three sessions per week of 4 hours each) (88) (see Chapters 8 and 10). Instead, in a multicenter, open-label, randomized controlled trial including 906 chronic HD patients and comparing the benefits of conventional versus high-efficiency postdilution HD, a reduction in all-cause mortality was associated with the latter; however, no difference in predialysis CRP values was found between the groups (89).
As cytokine-inducing substances present in the dialysis fluid may penetrate intact dialyzer membranes, impure dialysate is yet another factor to bear in mind (90). Indeed, Schindler et al. (91) have shown that small bacterial DNA fragments in dialysis fluid can pass through dialyzer membranes. In accordance, Sitter et al. (92) showed that a switch from conventional to online-produced ultrapure dialysate resulted in lower bacterial contamination with a significant decrease in CRP and IL-6 levels. Likewise, Shiffl et al. (93) showed that changing from conventional to ultrapure dialysate reduced the levels of IL-6 and CRP and improved nutritional status. Small studies have demonstrated that ultrapure dialysate is related to a slower loss of residual renal function (94) and a lower cardiovascular morbidity (95) in HD patients. Furthermore, vascular access is fundamental in HD patients, and an appropriate manipulation of it has a major impact on the prevalence of inflammation at the dialysis unit. Both clotted access grafts (96) and catheter infections (97) are significant contributors to the inflammatory process in HD patients. Biofilm formation might be also a cause of inflammation in this patient group (98). In summary, while adequate dialysis treatment to some extent can ameliorate some uremia-related proinflammatory factors, there are many dialysis-related factors that can contribute to the inflammatory state observed in CKD.
Additional Causes of Inflammation in Patients Undergoing Peritoneal Dialysis
The PD procedure per se may also induce systemic inflammation. Conventional bioincompatible glucose-based PD solutions, for instance, are thought to be important contributors (99), especially due to their content of glucose degradation products (GDPs) generated during heat sterilization. GDPs have been shown to induce peritoneal inflammation and the formation of AGEs. Additionally, glucose-based solutions lead to a substantial uptake of glucose, which may induce oxidative stress, a potent cause of inflammation (100). Peritoneal transport status is another important factor to consider in PD patients. Several years ago, it was suggested that patients presenting high peritoneal transport rate have worse clinical condition characterized by worse nutritional status and inflammation (101). However, it is now thought that the worse clinical situation and the high risk of death observed in the group of high transporters may be more closely related to volume overload from inadequate drainage in the exchanges. As volume overload occurs frequently in PD patients (78), this may be another reason for immune activation in this patient group. Additionally, concomitant infections especially peritonitis and percutaneous PD catheter infections are important contributors to inflammation in PD patients.
Activation of Polymorphonuclear Leukocytes
Although polymorphonuclear leukocytes (PMNLs) under noninfectious conditions are quiescent and release little ROS, there are reports that in the uremic milieu, PMNLs are primed long before RRT is initiated (FIGURE 20.3) (102). A study by Sela et al. (102) showed that PMNL priming as well as neutrophil counts were directly related to severity of kidney disease. When patients with CKD start dialysis treatment, PMNL priming seems to be further augmented (103). As PMNL priming is believed to be one important contributor to systemic oxidative stress and chronic low-grade inflammation in CKD (102), the characteristics of priming agents in the uremic milieu need to be elucidated. Although most uremic retention molecules have not yet been characterized with regard to their specific pro- and/or anti-inflammatory effects, some interesting observations have recently been reported. Glorieux et al. (104) showed that genuine AGE compounds activate the leukocyte response in the uremic condition. Moreover, the same group recently showed that p-cresylsulphate (the main product of the protein-bound uremic retention solute p-cresol) activates leukocyte free radical production (105). As p-cresol recently was shown to predict mortality in HD patients (106), the potential proinflammatory and proatherogenic effects of p-cresylsulphate need further consideration.

Causes Related to Comorbidity and Lifestyle
Comorbidities and unhealthy lifestyle issues like proinflammatory diets (107) and sedentary behavior (108) can contribute and potentiate the degree of inflammation and deserve special attention.
Oral Health and Periodontal Disease
It is appreciated that oral health is an important aspect in many chronic diseases. In CKD patients, diverse changes in the oral cavity like xerostomia and modifications in the microbial community are seen (109). These conditions together with the impaired immunity, poor oral hygiene, and malnutrition enhance the incidence of periodontitis and other manifestations of poor oral health (110). Although oral cavity health is often overlooked, poor oral health in CKD patients has been associated with systemic consequences as infections, PEW, and atherosclerotic complications (111). Moreover, several studies have shown that patients with periodontitis have elevated levels of CRP, and periodontal disease is thought to be an important contributor to local and chronic systemic inflammation in CKD (112). Given that oral health problems could constitute a permanent source of inflammation, poor dentition and other signs of poor oral health should be an alarm clock also at early stages of CKD. However, it remains to be determined whether more successful management of poor oral health and periodontitis will reduce the risk of inflammation, infections, PEW, and atherosclerotic complications in CKD.
Bowel Bacteria Overgrowth and Altered Gut Microbiota
Uremia results in profound alterations of the gut microbial flora, also called gastrointestinal dysbiosis, and provokes impairment in the structure and function of the intestinal epithelial barrier structure (113). The causes responsible for these derangements are related not only to uremia per se but also to other commonly observed conditions like fluid overload, dietary fiber intake, frequent use of antibiotics, slow colonic transit, metabolic acidosis, intestinal wall edema, and oral iron intake. Alterations of the gut microbial flora in CKD are related to systemic inflammation and accumulation of gut-derived uremic toxins. Some of the uremic toxins generated by colonic bacteria include alfa-phenylacetyl-l-glutamine, 5-hydroxyindole, indoxyl glucuronide, p-cresol sulfate, and indoxyl sulfate (114). It is thought that they might play a role in the progression of kidney disease and in the pathogenesis of accelerated CVD and numerous other CKD-associated complications (115). In this sense, using pre/probiotics in order to attenuate the imbalance in the microbiota has been proposed as an intervention to minimize inflammation in this group of patients (116). It also has been described that beside the effect of the composition of the intestinal microbiota, uremia leads to an altered function of the intestinal barrier by provoking increased intestinal permeability (117,118). As a consequence of the disrupted intestinal epithelial junctions, an increased translocation of bacterial products across the intestinal barrier is observed, leading to an activation of innate immunity and therefore yielding to systemic inflammation (113).
Obesity and Fat Mass
Obesity, a common feature of CKD, may also contribute to an enhanced inflammatory activity (119). Adipokines and proinflammatory cytokines have a tightly link to fat and muscle tissue (120). Considering the important effect that loss of renal function has on the clearance of these substances (121), the systemic effects of adipokines in patients with CKD appear to be greater than in the general population. It has been estimated that approximately 20% of circulating IL-6 originate from fat tissue, and a significant amount of the circulating TNF comes from macrophages resident in the adipose tissue (122). As visceral fat appears to produce adipokines more actively than subcutaneous adipose tissue, visceral abdominal fat may be a main producer of IL-6. In accordance, in CKD patients evaluated shortly before the start of RRT, a significant association between serum IL-6 and truncal fat, but not between IL-6 and nontruncal fat, was documented (123). This association has also been seen in nondialyzed CKD stage 3 to 5 patients, where increased amount of visceral fat associated with higher inflammatory parameters (124) and increased coronary artery calcification (125). Adding to the importance of fat mass distribution, rather than fat mass as such, in the same cohort, patients with more epicardial adipose tissue presented higher levels of inflammatory parameters as well as higher risk or CVD events (126).
Lifestyle Factors
Many lifestyle factors have been reported to potentiate the inflammatory status in CKD patients. Diet is a source of both anti- and proinflammatory constituents. In a study analyzing two community-based cohorts of elderly individuals, it was demonstrated that a proinflammatory diet is associated with systemic inflammation as well as with reduced kidney function (127). In this regard, dietary habits and food patterns are probably a target of major relevance in modifying systematic inflammatory levels. Moreover, both dialyzed and nondialyzed CKD patients have been reported to have a sedentary lifestyle that associate with higher values of inflammatory markers (108,128). Additionally, the implementation of programs devoted to increase physical activity in this group of patients has shown improvement in the levels of inflammatory parameters (129).
Obstructive sleep apnea syndrome is a common complication in patients with CKD (130) associated with endothelial damage, inflammation, and oxidative stress, which constitute an independent risk factor for CVD (112). Tobacco is probably the single most significant source of toxic chemical exposure to humans. Smoking is a central factor in many pathologic conditions, and its role in neoplasm, lung, and CVD has been well established for years. In recent years, cigarette smoking was shown to be able to alter both innate and immune systems and augment the production of numerous proinflammatory cytokines such as TNF, IL-1, IL-6, IL-8 GM-CSF, and a decrease in the levels of anti-inflammatory cytokines like IL-10 have been related to this (131,132).
Genetic Predisposition
Phenotypic variation is traditionally divided into a genetic and an environmental component. Undoubtedly, variations in the genome play an important role for the development of specific phenotype in CKD (133,134). Different types of genetic variants, such as insertion/deletions, mini- and microsatellites, or single nucleotide polymorphisms (SNPs), lead to a great variability in human genome, making human beings such unique individuals (134). Our understanding on the genetic predisposition to inflammation in patients with CKD has increased enormously the last decade. Because genetic variations are randomly assorted during gamete formation (independent of environmental factors), this approach, although being an association study, allows distinguishing a cause from an effect. For instance, the IL-6 gene has functional variants that affect inflammation and risk for CVD among dialysis patients, supporting a causal role for IL-6 in CVD (135). Interestingly, the IL-6 gene variants, together with those from the lymphotoxin-α gene, independently predicted risk for CVD in a rather big cohort of dialysis patients (136). Moreover, genetic variations in the IL-6 gene seem to influence inflammatory and peritoneal transport parameters, thereby contributing to the interpatient variability in small solute transport rate at the start of PD (137). Also, genetically determined interindividual differences in TNF (138), IL-10 (139), myeloperoxidase (140), and peroxisome proliferator-activated receptor (PPAR) γ (141) release have been associated with the prevalence of inflammation, CVD, and survival in CKD. Zhang et al. (142) found that although a haplotype consisting of several SNPs in the CRP gene was related to CRP levels measured over time in African American dialysis patients, there was no association between these SNPs and the presence of CVD. This finding is consistent with the growing belief that although CRP is a strong and independent risk marker of cardiovascular death, it is not a risk factor for vascular disease.
External and internal environmental stresses may also affect the phenotype via changes in the epigenome. Aberrant DNA methylation may, in relation to uremic dysmetabolism, have complex interactions for the development of premature CVD. As epigenetic mechanisms regulating the functional properties of the genome are heritable through cell divisions and may be sensitive to an abnormal environment (such as the uremic milieu), they could be potential new targets for interventions. Lastly, shortening of telomeres (nucleoprotein complexes protecting the chromosome ends that are involved in chromosome stability and repair) has been associated with an inflammatory phenotype and increased mortality in HD patients (143). In this context, it is of interest that telomere shortening to a critical length results in loss of histone and DNA methylation at mammalian telomeres, concomitant with increased histone acetylation (144). To conclude, the inflamed uremic phenotype is also the result of genetic factors. This is supported by the observation that Asian dialysis patients treated in the United States also have a markedly lower adjusted relative risk of mortality than Caucasians (145). Indeed, a substantial heritability (35% to 40%) has been found for CRP and IL-10 production (146,147), and many studies demonstrate a significant impact of genetic variations on the uremic inflammatory response (148,149).
Markers of Inflammation and Implications on Outcome
Prospective studies in patients both on HD (150–152) and PD (153–155) and after kidney transplantation (156) show that even a single measurement of an inflammatory biomarker is an independent predictor of poor outcome in patients with advanced CKD. In addition, results from the Modification of Diet in Renal Disease (MDRD) study showed that elevation of CRP (3 mg/L or more) and hypoalbuminemia predicted outcome in patients with mild to moderate CKD (157). In this regard, based on the large body of evidence available about the association of inflammation and poor outcomes in CKD population, an increased awareness of the importance of regular monitoring of inflammatory status has emerged in the nephrology community. For this purpose, a wide array of measurements are available (158), including among others, CRP, PMNL count, serum albumin, pentraxin-3 (PTX-3), IL-6, and TNF-like weak inducer of apoptosis (TWEAK) to name a few. However, there is poor agreement regarding which is the parameter that best predicts all-cause and cardiovascular mortality in patients with CKD.
In clinical practice, CRP is the most commonly used inflammatory marker. CRP is an acute-phase protein, produced by human hepatocytes in response to proinflammatory cytokines. Its measurement is cheap, reliable, easily obtained, and widely used in many centers around the world, and its interesting profile of features [stability over time (159), unaffected by circadian variation and by food intake] facilitates the use of it in dialysis patients attending different HD schedules. The use of CRP in dialysis units around the world has increased considerably in the last decade according to data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) registry (160), and, interestingly, the dialysis facilities with higher percentage of patients with available CRP measurements reported lower cardiovascular-related mortality. In the uremic milieu, CRP levels have shown to be associated with a number of both traditional and nontraditional cardiovascular risk factors, including dyslipidemia, oxidative stress, homocysteine levels, endothelial dysfunction, insulin resistance, and vascular calcification (26). A disadvantage faced with the use of CRP is its intra- and interindividual variability (161) and its variation over time, that in dialysis patients depend mainly on clinical events like transient infections, changes in volume status, or the intermittent stimulus of the dialysis procedure (162). Additionally, there is scarce information regarding the time-dependent predictive value of this measurement in patients with mild to moderate CKD. Moreover, whereas serial CRP measurements predict outcome better than a single CRP measurement in a group of prevalent HD patients (163), no data on serial determinations of inflammatory biomarkers are yet available in mild to moderate CKD. Therefore, prospective studies with careful serial monitoring of inflammatory biomarkers in patients with CKD are needed to evaluate if such an approach will yield more precise information to assess the disease severity.
IL-6 is considered as the key factor in the acute-phase response, and it has been suggested that this proinflammatory cytokine plays a strategic role in the pathogenesis of both PEW and atherosclerosis in the dialysis population (164). Some studies have assessed the predictive value of IL-6 over other inflammatory markers in dialysis patients, finding that IL-6 present the highest predictive value with regard to all-cause and cardiovascular mortality (34,35). Likewise, a comparative study based on ROC analysis also showed that the prediction power of the combined inflammatory burden of a number of commonly measured cytokines and adhesion molecules was identical to that provided by the sole measurement of IL-6 (165). Notwithstanding this advantage of risk prediction showed by IL-6, in a recent meta-analysis, Zhang et al. (166) found that CRP versus IL-6 had a similar predictive value for cardiovascular and all-cause mortality; CRP [hazard ratio (HR) 1.14 and 1.18, respectively] and IL-6 (HR 1.15 vs. 1.18, respectively). Thus, based on available data, it seems that the prognostic estimation provided by CRP is not much inferior to that by IL-6. Thus, the use of CRP would be sufficient to grade the inflammation status in clinical practice.
Other inflammatory markers that have attained increased interest in recent years are pentraxins. Pentraxins are a superfamily of evolutionarily conserved proteins characterized by a cyclic multimeric structure (167). On the basis of the primary structure of the subunit, the pentraxins are divided into two groups: short pentraxins (e.g., CRP and serum amyloid P) and long pentraxins. The prototype protein of the long pentraxin group is PTX-3. Whereas CRP and serum amyloid P are produced primarily in the liver in response to IL-6 (168), PTX-3 is produced by a variety of tissues and cells, particularly by innate immunity cells in response to proinflammatory signals and endothelial cells (169–171). Because of the extrahepatic synthesis and in contrast to CRP, PTX-3 levels are believed to be a true independent indicator of disease activity, produced at sites of inflammation and intimately linked to endothelial dysfunction (172). PTX-3 is elevated in HD patients as compared to healthy individuals (173) and identified as a novel mortality risk factor in CKD stage 5, independent of traditional risk factors, but most importantly independent of CRP itself (174). This suggests that this protein may have an additional role in the atherogenic process to common inflammatory mediators, perhaps reflecting endothelial damage.
Another novel inflammatory biomarker is TWEAK, (TNFSF12) is a member of the TNF superfamily of structurally related cytokines (175) that can be expressed as a full-length membrane-bound protein or as a soluble protein (sTWEAK) that results from proteolysis of TWEAK (176,177). TWEAK gene is expressed in many tissues, including brain, kidney, heart, arterial wall, monocytes, and macrophages, and it has been found to be involved in different biologic processes, such as induction of cellular growth and proliferation (178,179), osteoclastogenesis (180), angiogenesis (181), and stimulation of apoptosis (182), favoring in that manner an inflammatory microenvironment. Moreover, TWEAK attenuates the transition from innate to adaptive immunity (183), activates nuclear factor-κB (NF-κB) signaling pathway, and induces the expression of different proinflammatory cytokines and cell adhesion molecules (184,185). In CKD, sTWEAK levels are significantly decreased compared to healthy subjects; however, high sTWEAK plasma levels have been reported to have additive effects to the high cardiovascular and all-cause mortality of HD patients with systemic inflammation (186).
 CONSEQUENCES OF INFLAMMATION IN THE CLINICAL SETTING OF UREMIA
CONSEQUENCES OF INFLAMMATION IN THE CLINICAL SETTING OF UREMIA
In the CKD population, persistent inflammation has been associated with several negative outcomes (71), including PEW, atherosclerosis, as well as increased cardiovascular and all-cause mortality (7,187,188). The specific pathways by which this association might be mediated are still not well known; however, it is thought that inflammation, beside its own direct effect, potentiates other known risk factors, like oxidative stress (189), insulin resistance (190), endothelial dysfunction (191), vascular calcification (192), bone mineral disorders (193), and depression (194). Additionally, inflammation may magnify the risk for poor outcomes via mechanisms related to self-enhancement of the inflammatory cascade and exacerbation of both the wasting and the vascular calcification processes (195).
Protein-Energy Wasting
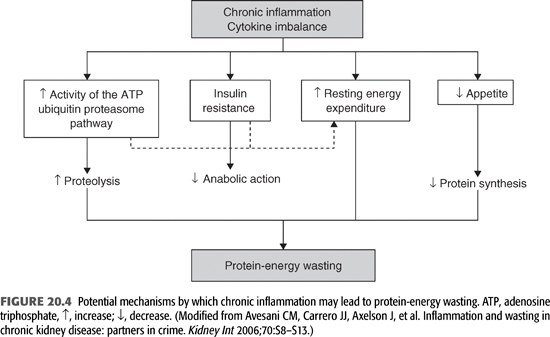
The term protein-energy wasting describes the loss of muscle mass and fuel reserves of the body (6). PEW is observed in as many as 18% to 75% of patients with CKD (196,197), and inflammation appears to be an important contributor of this complex syndrome, both by direct and indirect mechanisms of muscle proteolysis and by impinging upon and magnifying other causes of PEW in a vicious circle. In several studies, inflammatory markers have continuously been related to markers of muscle mass (198), indicating an important role of cytokines in the development of PEW and muscle catabolism (199). Muscle mass was inversely correlated to both IL-6 and CRP in HD patients, even after adjustments for age and gender (10). Also, markers of decreasing muscle mass during a 1-year period on HD were associated with higher IL-1β concentrations (200). Besides that skeletal muscle–derived IL-6 may contribute to the production of IL-6 and oxidative stress during the HD session (201,202), IL-6 per se may stimulate muscle protein breakdown and promote cancer-related wasting (203,204) and wasting observed in uremic patients (198). On the other hand, while IL-6 inhibits the secretion of insulin-like growth factor 1 (IGF-1), decreased IGF-1 signaling may also be involved in the sarcopenia process (205). Some of the known related mechanisms between PEW and inflammation are described below (FIGURE 20.4).
Anorexia
Anorexia represents a complex and multifactorial disorder with a high prevalence among CKD patients (206). Although anorexia is a typical consequence of CKD, other metabolic abnormalities that are not fully corrected by dialysis therapy are also involved. In these derangements, given that some inflammatory markers like CRP, IL-6, and TNF have been described as related to uremic anorexia (207,208), systemic inflammation is likely to play an integrative role in the physiopathology of anorexia in CKD. Inflammatory cytokines have the capacity to regulate the appetite through disturbing specific brain areas related to appetite regulation (209). Specific cytokines access the brain and act directly on hypothalamic neurons and/or generate mediators targeting both peripheral and/or brain target sites (209,210); thus, inflammation may influence the size, duration, and frequency of meals.
A difference in the relation between inflammation and anorexia among genders needs to be pointed out. A recent study showed that among CKD patients, anorectic women exhibited a more favorable inflammatory and nutritional status than anorectic men (155). This difference where apparently uremic men are more prone to inflammation-induced anorexia might be explained by a possible protective role of sex hormones against the burden of inflammation. Indeed, gender is physiologically associated with feeding behavior (158) and inflammatory status (159), and men with inflammation on dialysis seem to have a worse survival as compared to women with the same conditions (5). Although the role of sex-specific regulation of feeding is unclear, increased anorectic signals and earlier satiety have been reported in men suffering from chronic illnesses (160,161), perhaps contributing to a different response pattern to anorexigenic diseases (such as heart failure and cancer) among men and women (162). An indirect support of this hypothesis is that novel anorexigenic agents, such as megestrol acetate or nandrolone decanoate, have a chemical structure similar to sex hormones.
Increased Insulin Resistance
An increased insulin resistance predisposes to a loss of muscle mass by decreasing the anabolic action of insulin. Type 2 diabetic HD patients have significantly increased skeletal muscle protein breakdown (211). Thus, dialysis patients and patients with diabetes mellitus had significantly accelerated loss of lean body mass (LBM) during the first year of RRT (212). The link between inflammation and insulin resistance occurs mainly through (a) a defect in insulin intracellular signaling pathways during inflammation, (b) induction of lipolysis by TNF, and (c) a decrease in the secretion of adiponectin caused by TNF (213). Additionally, the administration of recombinant TNF to cultured cells or to animals impairs insulin action, and obese mice lacking functional TNF or TNF receptors have improved insulin sensitivity compared with wild-type counterparts (214).
Activation of the Adenosine Triphosphate Ubiquitin-Proteolytic System
Metabolic acidosis is a common phenomenon in progressive CKD that may lead to stimulation of protein breakdown and subsequent muscle wasting through stimulation of the ATP-ubiquitin-proteolytic pathway (215). Because TNF increases ubiquitin gene expression in skeletal muscle, it is possible that it causes muscle wasting by stimulating protein catabolism through the ubiquitin proteosome pathway (215,216).
Increased Resting Energy Expenditure
The diverse metabolic abnormalities present during the inflammatory response, like fever, elevated oxygen consumption, enhanced lipolysis and fat utilization, increased concentration of catabolic hormones, and extensive protein catabolism, consume high quantities of energy that account for as much as 15% of the daily energy expenditure (217). Studies in predialysis and dialysis patients have consistently shown that inflammatory markers are associated with increased resting energy expenditure (10,218,219) and an increased mortality risk (10).
Depression
Depressive symptoms are related to poor outcomes and higher mortality in CKD and in other patient groups, and they are seen more frequently with the gradual reduction in renal function (220–222). Cytokines are thought to be important mediators of brain immune connections and may play an important role in the pathogenesis of depression due to their effect on neurotransmitters and neurohormones (220,223). In dialysis patients, depressive symptoms seemed to worsen in the presence of increased IL-6 levels (222–226), and 8 weeks of fluoxetine treatment in depressed HD patients decreased serum IL-1β levels (227). Depression may undeniably link to fatigue (228) and unwillingness to eat (206), contributing in a vicious circle to anorexia, physical inactivity, PEW, and worse outcome, all of which have also been attributed, in part, to the effects of systemic inflammation.
Vascular Calcification
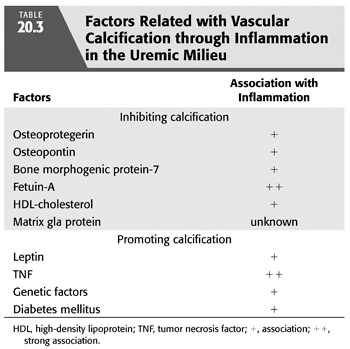
Although vascular calcification (or rather ossification) can be observed in the general population (229), CKD is a condition associated with a markedly increased prevalence of both intima and media arterial calcification (230). Indeed, several studies indicate that large artery calcification (assessed by computer tomography or chest x-ray) is present in 30% to 70% of patients with CKD (231,232), and even in approximately 15% of uremic pediatric patients (233).The presence of arterial ossification is associated with functional estimates of arterial dysfunction, such as NO-dependent vasodilation in dialysis patients (234) and pulse wave velocity (235), both of which have been associated with adverse outcome in patients with CKD (236,237). Indeed, vascular ossification is associated with increased risk of CVD (231,238,239) and mortality (238). Currently, there are a wealth of data evidencing intimate links between vascular calcification and systemic inflammation (TABLE 20.3). TNF can induce mineralization of calcifying vascular cells in vitro (240), and coculture of these cells with monocyte/macrophages (the source of most cytokines) can accelerate this process (241). Receptor activator of NF-κB ligand (RANKL) is a membrane-bound or soluble cytokine essential for osteoclast differentiation, whereas the decoy receptor osteoprotegerin (OPG) masks RANKL activity. Although both seem to influence the inflammatory component of atherosclerosis (242), it is of interest that OPG upregulates endothelial cell adhesion molecule in response to TNF (243). These findings suggest a mechanism by which OPG may stimulate inflammation in atheroma and thereby promote the progression and complications of atherosclerosis, which would agree with the observed detrimental effects on survival of both increased inflammation and OPG levels in HD patients (244). On the other hand, vascular calcification, as part of the atherosclerotic process, is due to the deposition in the arterial intima of basic calcium phosphate (BCP) crystals, similar to those that mineralize bone. It was recently shown that BCP crystals could interact with human monocyte-derived macrophages, inducing a proinflammatory state through protein kinase C and mitogen-activated protein (MAP) kinase pathways (245). This implies a vicious circle of inflammation and arterial calcification that could explain the associations between inflammation and outcome in CKD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


