Hysteroscopy for Infertility
Charles M. March
From its humble beginnings almost 150 years ago, hysteroscopy has become a sophisticated procedure that is invaluable in the management of certain diseases that cause infertility and recurrent spontaneous abortion. The instruments to be considered in this chapter are the panoramic hysteroscope, the contact hysteroscope, the microhysteroscope, and the steerable, or flexible, hysteroscope. Each of these has advantages and disadvantages in the management of the woman who has an acquired or congenital uterine disease and who suffers from reproductive failure.
Hysteroscopy and Hysterosalpingography
Hysteroscopy has been recommended as a procedure to replace hysterosalpingography (HSG). However, hysteroscopy and HSG should be considered complementary rather than competing techniques. First, HSG is a relatively inexpensive procedure that provides important information about the endocervical canal, the region of the internal os, the uterine cavity, and the entire courses of the fallopian tubes. For the infertile patient, the latter information is invaluable. Second, HSG details the geometry of the uterine contour more clearly than is possible with hysteroscopy and is superior to hysteroscopy in the detection of adenomyosis. Finally, HSG may reveal information that would alter the patient’s management. The finding of very large hydrosalpinges not amenable to reconstructive surgery may cause the workup to be discontinued and referral made for in vitro fertilization and embryo transfer. An illness such as pelvic tuberculosis causing bilateral tubal obstruction may be suspected initially only because of classic radiographic findings. Although a most unusual condition in the United States and western Europe, patterns of travel and immigration, an increase in the number of women with immune deficiency disorders, and a rise in the resistance of the organism to first-line treatment agents have caused an increase the frequency of pelvic tuberculosis. In this instance other studies (endometrial biopsy, culture of menstrual effluent) would be performed, and if the diagnosis were confirmed, the infertility workup would be terminated.
A comparison of HSG and hysteroscopy is shown in Table 28.1. If hysteroscopy is performed in the office without anesthesia or with local anesthesia only, the cost of hysteroscopy is the same as that of HSG. Hysteroscopy in the office offers a plethora of advantages to both patient and physician compared with hysteroscopy in a surgery center or at a hospital under general anesthesia (Table 28.2). Table 28.1 demonstrates clearly the advantages of hysteroscopy over HSG. However, as a screening procedure and because of the additional information it provides, HSG should not be abandoned. Some authors have suggested that hysteroscopy will frequently reveal lesions in infertile patients with infertility and in those with recurrent abortion whose hysterosalpingograms are normal. However, the nature and the significance of these findings is suspect. Others have indicated that hysteroscopy may be used to predict the success of in vitro fertilization. If recent HSG demonstrates a normal endometrial cavity (including views taken early during the uterine filling phase) and, provided that the axis of the uterus is parallel to the film plate, hysteroscopy need not be performed. However, if HSG suggests the presence of an intrauterine defect, hysteroscopy is mandatory so that the presence of the lesion may be confirmed and its nature determined with almost 100% certainty.
Indications
Although the hysteroscope is an important instrument for the diagnosis of some causes of infertility, its main value lies in that of treatment. Intrauterine diagnosis and surgery for the patient with reproductive failure should be confined to the procedures listed in Table 28.3. Although hysteroscopy has been used successfully to evacuate ectopic pregnancies in the cervical canal, in cesarean section scars, in the intramural portion of the oviduct, and in a number of other conditions related to reproductive performance, most of these are isolated case reports and do not have widespread application. Of the indications listed in Table 28.3, hysteroscopy for both the diagnosis and treatment of intrauterine adhesions (IUA) was the first procedure proven to be superior to older techniques. Simultaneous hysteroscopy and laparoscopy to treat the septate uterus has made the Tompkins and Jones procedures obsolete. However, this anomaly is more likely to cause pregnancy loss than infertility. The data to support the use of hysteroscopy to resect submucous myomas are less plentiful, but have provided ample evidence of the value of hysteroscopy in selected cases. Polyps were generally not considered to be the
cause of infertility or repetitive pregnancy loss but newer data suggest otherwise. Often polyps cannot be diagnosed with certainty by HSG, and hysteroscopy permits their complete removal (compared with curettage) with less endometrial trauma than that caused by curettage. Cannulation of proximally obstructed fallopian tubes by way of their ostia is very successful and has certain advantages over the radiographic approach. In the past, transcervical transfer of gametes and embryos under hysteroscopic guidance had shown promise in the hands of a few investigators. However, the success rates of blind embryo transfer (ET) or ET under ultrasound guidance have obviated the need for hysteroscopy in in vitro fertilization–embryo transfer (IVF-ET).
cause of infertility or repetitive pregnancy loss but newer data suggest otherwise. Often polyps cannot be diagnosed with certainty by HSG, and hysteroscopy permits their complete removal (compared with curettage) with less endometrial trauma than that caused by curettage. Cannulation of proximally obstructed fallopian tubes by way of their ostia is very successful and has certain advantages over the radiographic approach. In the past, transcervical transfer of gametes and embryos under hysteroscopic guidance had shown promise in the hands of a few investigators. However, the success rates of blind embryo transfer (ET) or ET under ultrasound guidance have obviated the need for hysteroscopy in in vitro fertilization–embryo transfer (IVF-ET).
TABLE 28.1 Comparisons of Hysteroscopy and Hysterosalpingography (HSG) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
On occasion, hysteroscopy is necessary to remove a retained foreign body, most commonly an intrauterine device (IUD). The routine use of hysteroscopy for all “missing string” IUDs is not advised. Rather, a limited role is more appropriate. Removal of retained products of conception after a spontaneous or induced abortion is a small but evolving role for hysteroscopy. This approach helps to reduce the extent of uterine trauma while ensuring that all retained tissue has been removed. The approach has special value in patients with uterine anomalies and in those with intracavitary myomas. In these instances many physicians choose to evacuate the uterus under ultrasound guidance. Although no comparative data are available to demonstrate that one technique is superior to the other, the need to visualize the uterine cavity directly or indirectly to reduce trauma and to ensure complete evacuation is clear. In some patients, retained products of conception may ossify many years later, and removal under hysteroscopic guidance is the treatment of choice.
TABLE 28.2 Advantages of Hysteroscopy in the Office under Local Anesthesia versus in a Surgery Center or Hospital | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 28.3 Hysteroscopic Procedures to Reproductive Performance | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
TABLE 28.4 Reproductive Performance in Infertile Patients with Untreated Intrauterine Adhesions (n = 292) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Intrauterine Adhesions
Etiology
The frequency of IUA is unknown, and although it is likely that some patients with IUA are asymptomatic and have normal fertility and reproductive performance, the overall reproductive performance in those with untreated IUA is dismal (Table 28.4). The etiology, sequelae, and manifestations of IUA are outlined in Table 28.5. The sine qua non for the development of IUA is endometrial trauma, especially to the
basalis layer. The pregnant or recently pregnant uterus is most vulnerable. Although uterine infection is usually described as an important contributing factor, clinical evidence of infection has been present in <1% of our patients. None of our patients who underwent curettage had clinical evidence of infection, and many had received “prophylactic” antibiotics. If the postpartum uterus is curetted, adhesions are most likely to develop if the procedure is performed between the second and fourth weeks after delivery. Among postpartum patients, concomitant breast-feeding increases the risk of adhesion formation. Women who nurse remain estrogen deficient for a prolonged period; thus, the stimulus to endometrial regeneration is missing. The same heightened risk would be present in those with Sheehan syndrome.
basalis layer. The pregnant or recently pregnant uterus is most vulnerable. Although uterine infection is usually described as an important contributing factor, clinical evidence of infection has been present in <1% of our patients. None of our patients who underwent curettage had clinical evidence of infection, and many had received “prophylactic” antibiotics. If the postpartum uterus is curetted, adhesions are most likely to develop if the procedure is performed between the second and fourth weeks after delivery. Among postpartum patients, concomitant breast-feeding increases the risk of adhesion formation. Women who nurse remain estrogen deficient for a prolonged period; thus, the stimulus to endometrial regeneration is missing. The same heightened risk would be present in those with Sheehan syndrome.
TABLE 28.5 Factors That Contribute to IUA Formation, the Sequelae of Endometrial Injury, and the Manifestations of Intrauterine Adhesions or Endometrial Sclerosis | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Polishuk and Sadovsky reported 11 patients with recurrent IUA. Some of these patients had undergone therapy for focal IUA in one area of the uterus and subsequently developed scarring elsewhere. Thus, they suggested that this condition represents a response to a generalized disorder such as myometrial fibrosis. This attractive theory is unproven. However, in those women who do conceive after therapy for IUA and who suffer another pregnancy loss, we strongly advocate medical rather than surgical evacuation of the uterus. If curettage is necessary, we advise placement of a stent and estrogen therapy after surgery. This recommendation applies to those in other high-risk groups, such as women who undergo curettage because of a delayed postpartum hemorrhage. Stillman and Asarkof reported an associated between müllerian anomalies and IUA. Although they seemed to suggest that the anomaly predisposed to adhesion formation, many of the patients had undergone multiple curettages because of repetitive pregnancy loss. At present there are no data to prove that the anomalous uterus is more prone to adhesion formation.
Retained Products of Conception
If the curettage is delayed long after fetal death, the risk of scarring increases. Recent data suggest that adhesions occur more often after curettage for a missed abortion than after curettage for a spontaneous, incomplete abortion. Adhesions were found after curettage in 13 (30.9%) of 42 women who had a missed abortion, compared with only 5 (6.4%) of 78 who had an “early” abortion. Schenker and Margalioth suggested that retained placental fragments might induce fibroblast activity and collagen formation prior to endometrial regeneration. Massouras reported that none of 170 patients who had sustained endometrial trauma (usually curettage) developed IUA if a specially designed IUD was used for prophylaxis. Although others have not confirmed his findings, prophylactic use of a splint together with estrogen therapy might be warranted in those who are at high risk for IUA formation. If products of conception are retained for many weeks after delivery, curettage, or fetal demise, complete extraction without inducing endometrial injury can be complicated because the tissue has usually become markedly adherent. Removal under ultrasound guidance or (ideally) under hysteroscopic control allows extraction efforts to be directed only to the areas where tissue remains. If a resectoscope is used, care should be taken to use a loop electrode without the application of energy if possible. Other causes of IUA are pelvic irradiation, endometrial tuberculosis, and septic abortion.
Most patients who develop uterine damage after curettage have scars that bridge the anterior and posterior uterine walls (Fig. 28.1). Almost always some viable endometrium is
interspersed between these scars, even if they are extensive. However, a variant of this disease is called endometrial sclerosis or an “unstuck” Asherman. This pathology is the most severe because it represents partial destruction of the basal endometrial layer. This type of pathology is seen after myomectomies, whether abdominal or hysteroscopic and following other types of intrauterine surgery during which laser or electrical energy had been used. It represents a partial endometrial ablation.
interspersed between these scars, even if they are extensive. However, a variant of this disease is called endometrial sclerosis or an “unstuck” Asherman. This pathology is the most severe because it represents partial destruction of the basal endometrial layer. This type of pathology is seen after myomectomies, whether abdominal or hysteroscopic and following other types of intrauterine surgery during which laser or electrical energy had been used. It represents a partial endometrial ablation.
Symptoms and Diagnosis
The manifestations of intrauterine adhesions include menstrual aberrations such as hypomenorrhea or amenorrhea, infertility, pregnancy wastage (including both first- and second-trimester abortions), missed abortion, intrauterine fetal demise, and errors of placental implantation (such as placenta accreta, increta, and percreta). Prior to the advent of hysteroscopy, the diagnosis of IUA depended on historical criteria, laboratory data, the response to hormonal stimulation, and HSG. Suspicion is aroused whenever there is a history of hypomenorrhea or amenorrhea following curettage. The pregnant or recently pregnant uterus is more vulnerable to injury than is the nonpregnant uterus. If the patient is amenorrheic but has cyclic changes that suggest normal ovarian function, the likelihood that she has adhesions is high. Studies used to document ovulation in these patients include a basal body temperature record and serial serum progesterone levels. If the patient is ovulating, the former will be biphasic but may look atypical because the initial values may not be obtained early in the follicular phase, because cycle day 1 will not be known. The progesterone determinations are obtained at weekly intervals until one value >3 ng/mL is obtained.
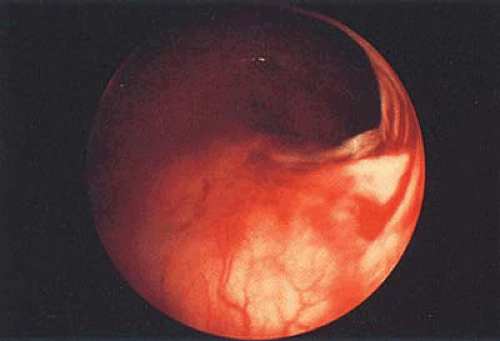
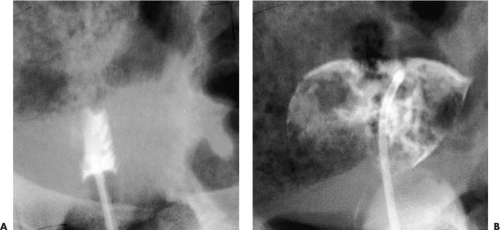
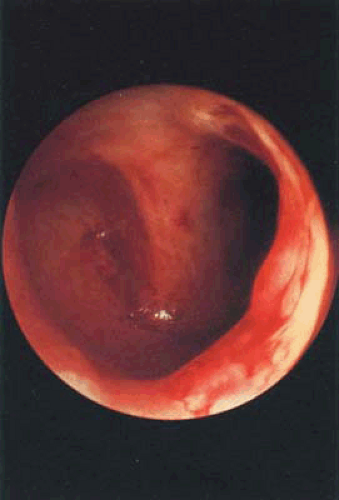
The failure to have withdrawal bleeding despite ovulatory cycles is strongly suggestive of the presence of IUA. Further studies to suggest the presence of IUA are: difficulty in sounding the endometrial cavity, the presence of fibrosis in an endometrial biopsy, and failure to bleed after the administration of a progestin or the sequential administration of an estrogen and a progestin. If HSG reveals single or multiple irregular filling defects, the diagnosis of IUA is relatively secure. However, because all of these criteria are occasionally positive in the absence of IUA, the diagnosis may be made with certainty only by direct inspection of the uterine cavity (Fig. 28.1). If HSG demonstrates no fill of the uterine cavity (Fig. 28.2A), a pelvic ultrasound may show hematometra indicating that there is outflow tract obstruction, and relatively normal endometrial function (Fig. 28.3). If HSG is performed immediately after disrupting the obstruction in a patient with hematometra, a bizarre image will be obtained because the contrast will outline multiple blood clots (Fig. 28.2B). This condition (scarring in the region of the internal os) is what Dr. Asherman described in most of the patients from his original report. Treatment has an excellent prognosis in these patients and should be considered even in those who do not wish to conceive in the future because persistent retrograde menstruation may lead to the development of endometriosis.
Therapy
After the diagnosis of IUA has been made, the treatment goals are to restore uterine architecture to normal, to prevent
readherence of the uterine walls, to provide stimulation for endometrial growth over the freshly dissected surfaces, to verify that the uterine architecture is normal, and to ascertain that the endometrial development in response to endogenous ovarian steroid production is normal prior to permitting the patient to attempt to conceive (Table 28.6). For those with IUA in whom tubal patency has been demonstrated or suspected, contraception should be used until the disease has been corrected because of the poor outcome of pregnancies in women with untreated or partially treated intrauterine synechiae.
readherence of the uterine walls, to provide stimulation for endometrial growth over the freshly dissected surfaces, to verify that the uterine architecture is normal, and to ascertain that the endometrial development in response to endogenous ovarian steroid production is normal prior to permitting the patient to attempt to conceive (Table 28.6). For those with IUA in whom tubal patency has been demonstrated or suspected, contraception should be used until the disease has been corrected because of the poor outcome of pregnancies in women with untreated or partially treated intrauterine synechiae.
Although there is no information to show that the results of early therapy for IUA are superior to those of treatment provided many years after the development of adhesions, most patients who have menstrual disturbances wish to be treated even if pregnancy is not desired in the near future. The woman who is not interested in pregnancy should be advised that she cannot use amenorrhea and the presumed presence of IUA as an effective contraceptive. For these reasons, most patients are treated shortly after the diagnosis has been made.
Results
Prior to the advent of hysteroscopy, treatment for IUA consisted of an attempt to disrupt the adhesions bluntly, using a uterine sound or small curette. When these blind approaches were used, many authors advocated simultaneous laparoscopy in an attempt to reduce the frequency of uterine perforation. If these methods failed, other procedures including endometrial transplants and hysterotomy were performed. Although between 50% and 90% of patients with menstrual disturbances had normal flow restored following these types of therapy, pregnancy rates remained quite low, that is, between 35% and 60%. Of even greater significance is the fact that <50% of these pregnancies resulted in a term viable infant. Moreover, between 20% and 35% of those women who were delivered at term had complications of placental implantation. Thus, blind approaches to the treatment of IUA were usually unsuccessful in achieving a good gestational outcome.
TABLE 28.6 Treatment Objectives for Patients with Intrauterine Adhesions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
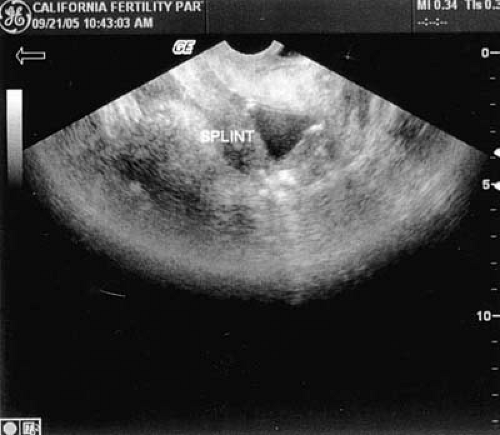
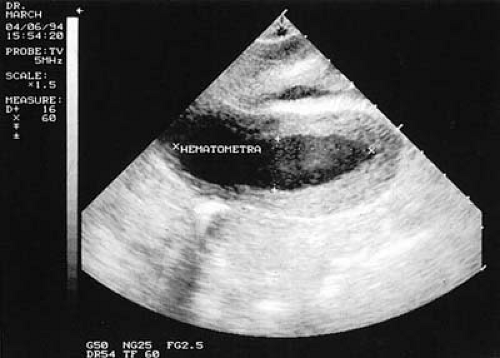 FIGURE 28.3 Ultrasound demonstrating hematometra in a patient with postcurettage amenorrhea and scarring of the lower uterine segment. |
Early hysteroscopic attempts by Sugimoto to treat IUA led to an overall pregnancy rate of about 40%, but <60% of these patients were delivered at term and the frequency of placental complications was 17.8%. This report was rather discouraging because despite the fact that diagnosis was made with certainty and despite the fact that most of the adhesions were being disrupted under visual control, the outcome was not better than that which had been achieved with earlier primitive techniques. The failure to achieve good pregnancy rates and the high rate of placental problems may be related to the technique used. Sugimoto used the outer sleeve of the hysteroscope to disrupt the adhesions bluntly. This technique is likely to be successful for most adhesions located in the center of the uterine cavity. However, those scars that are present along the sidewalls of the uterus and at the top of the fundus—that is, so-called marginal adhesions—could not be lysed using this technique. Therefore, he used Kelly forceps to disrupt the adhesions after they had been localized with the hysteroscope. Again, a blind technique was being used. It is likely that this approach led to either excessive endometrial trauma or incomplete lysis of adhesions. For these reasons, the fertility rate, gestational outcome, and the complications of pregnancy were worse than might be expected from a hysteroscopic approach.
Table 28.7 provides data on our first 636 patients who underwent treatment for IUA. These patients ranged in age from 19 to 42 years. Although 614 of them had been pregnant on one or more occasions, only 399 had delivered an infant. In this group of patients, the most common antecedent factor was curettage for a voluntary pregnancy termination. Most of these procedures were performed by a suction technique, which was followed frequently by a sharp curettage. Curettage for an incomplete abortion was the second most common
cause, and other causes included curettage for postpartum hemorrhage and a diagnostic dilatation and curettage (D&C). Others have reported the risk of developing IUA after a diagnostic curettage. With the realization that even a diagnostic D&C can result in adhesions, it would be prudent to refrain from the routine use of D&C at the time of diagnostic laparoscopy for infertility. If performed during the follicular phase of the cycle, this procedure does not provide useful information. If the laparoscopy is done during the luteal phase and if endometrial dating is the goal of endometrial sampling, an adequate histologic sample may be obtained by an endometrial biopsy, and full curettage is not necessary. Unusual antecedent factors for the development of IUA are cesarean section, metroplasty, and myomectomy. In three of our patients, no antecedent factor could be detected.
cause, and other causes included curettage for postpartum hemorrhage and a diagnostic dilatation and curettage (D&C). Others have reported the risk of developing IUA after a diagnostic curettage. With the realization that even a diagnostic D&C can result in adhesions, it would be prudent to refrain from the routine use of D&C at the time of diagnostic laparoscopy for infertility. If performed during the follicular phase of the cycle, this procedure does not provide useful information. If the laparoscopy is done during the luteal phase and if endometrial dating is the goal of endometrial sampling, an adequate histologic sample may be obtained by an endometrial biopsy, and full curettage is not necessary. Unusual antecedent factors for the development of IUA are cesarean section, metroplasty, and myomectomy. In three of our patients, no antecedent factor could be detected.
TABLE 28.7 Antecedent Factors in 636 Patients with Proven Intrauterine Adhesions | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 28.8 Menstrual Patterns in 636 Patients with Proven Intrauterine Adhesions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Among our patients the most common menstrual pattern was that of amenorrhea; 114 other patients complained of hypomenorrhea, and 8 had oligomenorrhea (Table 28.8). Those with oligomenorrhea were treated with clomiphene citrate and resumed normal menstrual patterns. Therefore, 49 of the 636 patients (7.7 %) had normal menstrual flow. Thus, the dictum that states that the occurrence of bleeding following the administration of progesterone in oil or the sequential administration of an estrogen and progestin rules out the diagnosis of IUA is not valid.
One great value of hysteroscopy is that it allows the operating surgeon to classify the extent of the disease (Table 28.9 and Fig. 28.4). By classifying adhesions under direct vision, their location, extent, and degree of vascularity of adhesions may be known with certainty. In those with minimal or moderate disease, the endometrium in areas not affected by synechiae may be assessed. The use of a uniform classification based on direct inspection of the cavity is superior to classification systems based on HSG and permits meaningful comparison of treatment techniques to be made.
TABLE 28.9 Hysteroscopic Classification of Intrauterine Adhesions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
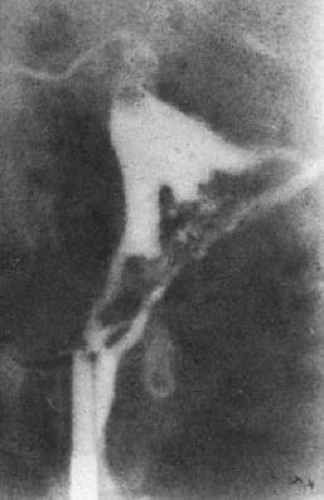
Figures 28.5, 28.7, 28.7 demonstrate the appearance of hysterosalpingograms showing minimal, moderate, and severe adhesions, respectively. In our series, 529 patients had HSG prior to undergoing hysteroscopy. There was fair correlation between the radiographic findings and those seen by hysteroscopy (Table 28.10). Frequently, HSG tended to exaggerate the extent of disease. The most dramatic example of this fact was in two women with endometrial sclerosis who had no adhesions, although HSG suggested complete obliteration of the endometrial cavity. However, in no instance did HSG demonstrate less severe adhesions than were found by direct uterine inspection. As would be expected, there was good correlation between the extent of the adhesions and the menstrual pattern (Table 28.11). The great majority of patients who had amenorrhea had either severe or moderate adhesions. However, it is important to note that 15 patients
who had moderate adhesions and 5 women who had extensive disease had either normal menses or oligomenorrhea.
who had moderate adhesions and 5 women who had extensive disease had either normal menses or oligomenorrhea.
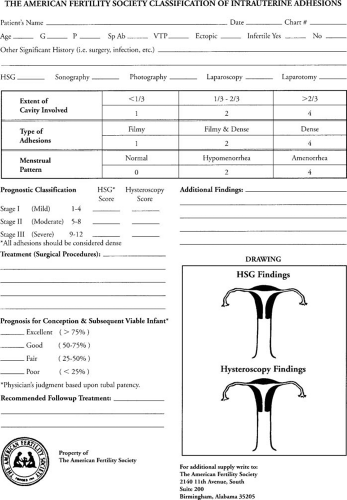 FIGURE 28.4 American Fertility Society Classification of intrauterine adhesions. (From The American Society for Reproductive Medicine, Birmingham, AL. ) |
TABLE 28.10 Correlation Between Extent of Adhesions by Hysterosalpingography (HSG) and Hysteroscopy in 529 Patients with Proven IUA | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Hysteroscopy was performed in the follicular phase for those patients who were menstruating. Approximately 4% of the procedures were done under local anesthesia using a paracervical block and conscious sedation. After adequate local or general anesthesia is obtained, the cervical canal is dilated and the hysteroscope, which has been prefilled with Hyskon or a sorbitol-mannitol solution, is inserted to the level of the external os and, if possible, advanced into the cavity under direct vision. If no landmarks are identifiable and if the hysteroscope cannot be advanced safely, laparoscopy is performed before further hysteroscopic efforts are made. Laparoscopy has been required in 20% of our patients. After the pelvis is inspected, the intensity of the light source for the laparoscope is reduced markedly. If the hysteroscopic adhesiolysis is carried inadvertently into the myometrium, the light from the hysteroscope will shine brightly through the uterine serosa, thereby affording the laparoscopist the opportunity to redirect the plane of the hysteroscopic dissection before uterine perforation occurs. Whether or not simultaneous laparoscopy is necessary, the uterine cavity is inspected and adhesions are identified (Fig. 28.8 and 28.9), and miniature scissors are passed through the operating port of the hysteroscope. Adhesions are lysed under direct vision (Fig. 28.10A, B). In three patients entry into the uterus was not
possible via the cervix and hysterotomy was necessary to gain entry into a minute uterine cavity. In these instances, adhesiolysis proceeds from superiorly to inferiorly.
possible via the cervix and hysterotomy was necessary to gain entry into a minute uterine cavity. In these instances, adhesiolysis proceeds from superiorly to inferiorly.
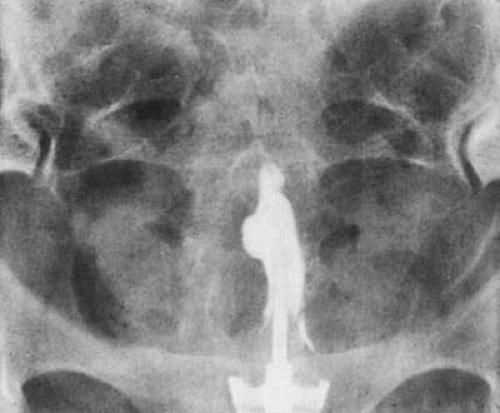 FIGURE 28.7 Hysterosalpingogram demonstrating filling of only the endocervical canal and a portion of the lower uterine segment in a patient with extensive intrauterine adhesions. |
TABLE 28.11 Correlation Between Presenting Menstrual Pattern and Extent of Adhesions Diagnosed by Hysteroscopy | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
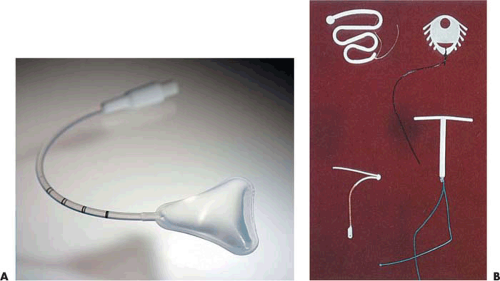
A Cook balloon uterine stent (Fig. 28.11A) is inserted into the uterine cavity. This device is triangular, and therefore its shape conforms to the configuration of the normal uterus. After placement the device is inflated, allowing it to expand and keep the uterine walls apart during the initial healing phase (Fig. 28.12). Its shape prevents the upper fundus and the cornual recesses, areas which are crucial to implantation, from being in apposition. This device has replaced a Lippes Loop IUD (Fig. 28.11B) as a means of reducing the chance of adhesions reformation. An inert device is preferred to the IUDs presently available in the United States. All of these have a relatively small “T” shape that affords inadequate coverage of the freshly dissected surfaces. The copper in the ParaGard IUD causes excessive intrauterine reaction, and the Mirena and Progestasert devices release progestins locally, thereby inhibiting endometrial proliferation. An estrogen-bearing IUD had shown some promise during initial animal studies, but human trials were not initiated. If the balloon uterine stent cannot be placed, a 12-16 french Foley catheter is inserted and inflated. The stent is retained for 5 to 14 days depending on the extent and density of the scars, and 100 mg doxycycline twice daily is prescribed during this time. Recently a hyaluronic acid gel has been used to prevent adhesion reformation after hysteroscopic surgery in women with IUA and to prevent de novo scar formation in women undergoing other types of intrauterine surgery with a resectoscope.
The second adjunctive therapy is high-dose estrogen therapy given to induce endometrial proliferation. All patients receive Estrace 3 to 4 mg daily for 30 to 60 consecutive days. On the last 5 days of the estrogen therapy, medroxyprogesterone acetate, 10 mg per day, is added. For patients whose endometria do not respond to estrogen stimulation, perhaps because of fibrosis of, or poor perfusion to, the subendometrial layer, medications such as aspirin, sildenafil citrate, and a combination of pentoxifylline and tocopherol have been prescribed with irregular success.
Following the drug-induced withdrawal bleeding, the patient’s cavity is inspected. The second investigation is
usually by hysteroscopy performed in the office using CO2 for uterine distention. However, if the initial procedure was very difficult, or if persistent adhesions are suspected, follow-up is by hysteroscopy in an operating room. Figure 28.13A–C demonstrates adhesiolysis using a laser fiber. Others prefer to use a resectoscopic electrode or a bipolar electrode. We believe that the use of these instruments is not necessary and may cause thermal injury in the presence of an endometrium that has been compromised already.
usually by hysteroscopy performed in the office using CO2 for uterine distention. However, if the initial procedure was very difficult, or if persistent adhesions are suspected, follow-up is by hysteroscopy in an operating room. Figure 28.13A–C demonstrates adhesiolysis using a laser fiber. Others prefer to use a resectoscopic electrode or a bipolar electrode. We believe that the use of these instruments is not necessary and may cause thermal injury in the presence of an endometrium that has been compromised already.
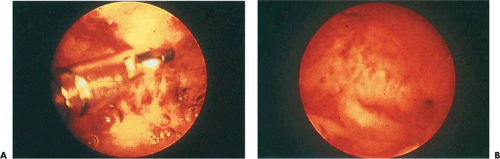 FIGURE 28.10 A: View of flexible hysteroscopic scissors dividing the adhesions pictured in Figure 28.9. B: View of uterine cavity immediately after lysis of the adhesions in Figure 28.9. The left tubal ostium is now visible. |
Protopapas et al. described a technique of “myometrial scoring” to treat patients who had severe scarring and whose prior attempts to normalize the uterine cavity by means of hysteroscopic adhesiolysis had failed. The small, cylindrical cavity is enlarged by means of making six to eight passes with a loop electrode into the myometrium. The hope is to enlarge the cavity and, most important, to uncover some islands of endometrium that may have been buried by prior surgical procedures. Little is known about the effectiveness
of this approach but because the effective therapy is lacking for some of the most difficult cases, the investigation of new techniques is warranted.
of this approach but because the effective therapy is lacking for some of the most difficult cases, the investigation of new techniques is warranted.
Of those patients who underwent a postoperative study of the uterus following one procedure, 85% had a normal cavity. However, some patients with extensive adhesions did require repeat procedures, and in one instance hysteroscopic therapy was carried out on five occasions before the uterus could be normalized (Figs. 28.14, 28.15, 28.16, 28.17). Overall, 90% of our patients had normal uterine anatomy restored. The etiology of IUA formation in two groups of patients deserves special consideration: those whose IUA follow a postpartum hemorrhage and those whose IUA develop after gynecologic surgery. Among those whose IUA followed a postpartum curettage, our “success” rate is much lower than that achieved in the overall group (approximately 50% vs. >95% in the postabortal group). If adhesions develop following myomectomy, especially if extensive hysteroscopic surgery preceded IUA formation, the prognosis is poor. Many of these patients have lost much of their basal endometrial layer and regeneration is not possible.
Some method of verifying that the uterine architecture is normal is mandatory prior to allowing the patient to conceive. It is likely that the poor gestational outcome that followed earlier treatment methods was secondary to the adverse effect of residual adhesions.
Of the patients who have wished to conceive and in whom no other infertility factors could be identified, 75% have done so. In Table 28.12 the gestational outcomes following so-called traditional methods of treatment and the results from four series following hysteroscopic therapy are compared. One patient in our series had placenta accreta, and one required manual removal of the placenta. One of the patients reported by Valle and Sciarra had placenta accreta also. Both of our patients who had placental problems had residual IUA demonstrated by follow-up HSG but conceived prior to undergoing repeat hysteroscopic adhesiolysis. Among our first 38 women who conceived, only 14 of 84 wanted pregnancies (16.7%) resulted in a term gestation prior to treatment for IUA. The corrected term pregnancy rate after treatment of these women was 87.2% (34/39) (Table 28.13). Other investigators have reported similar results (Table 28.13). Thus hysteroscopy permits the diagnosis of IUA to be established with certainty and the extent of the disease to be classified. It also permits complete lysis of adhesions. This safer, more accurate technique results in a gestational outcome that surpasses that achieved by earlier therapeutic regimens and has replaced all other treatment methods for the management of IUA.
Three causes of IUA remain resistant to therapy: the postpartum curettage, especially if the patient was hypoestrogenic; the abdominal myomectomy; and extensive intrauterine surgery. Thus, the challenges are to anticipate these injuries, to reduce their frequency and severity, and to develop techniques that facilitate early diagnosis.
Leiomyomas and Endometrial Polyps
Symptoms and Diagnosis
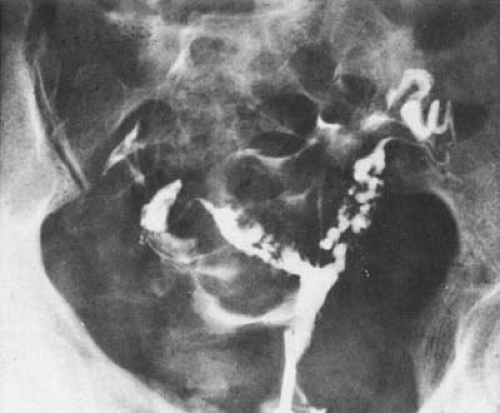
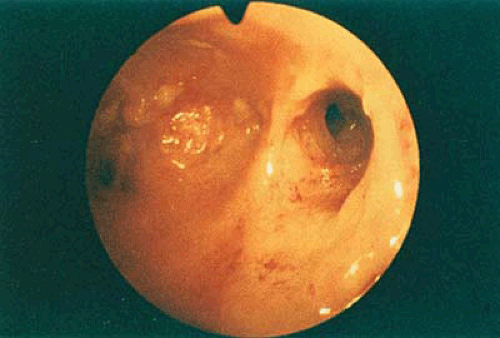
For the infertile patient who has a cavity defect demonstrated by HSG (present in about 10% of infertile patients), hysteroscopy allows the diagnosis to be made with certainty. For the woman with an endometrial polyp (Figs. 28.18, 28.19, 28.20, 28.21), lesions that cannot always be differentiated from submucosal myomas by HSG (Figs. 28.22 and 28.23), hysteroscopy is invaluable (Figs. 28.24). Moreover, under direct vision, the hysteroscope allows the surgeon to resect the polyps completely without traumatizing the adjacent normal endometrium. This is especially important in the patient who has multiple polyps (Fig. 28.25). Figure 28.26 shows an endometrial polyp in a patient who presented with excessive uterine bleeding and who had undergone curettage on six occasions. It was resected easily by hysteroscopy.
The infertile patient with one or more submucosal myomas may also complain of pregnancy wastage. As with polyps, a definitive diagnosis cannot always be made by a hysterosalpingogram (Figs. 28.2 and 28.27). Under direct vision the surgeon can evaluate the nature of the cavity defect and be certain of its extent and proximity to other important structures such as the internal os or a tubal ostium (Figs. 28.28, 28.29, 28.30). Following the initial description by Neuwirth, other investigators have demonstrated the value of hysteroscopy for the treatment of submucosal myomas. If the degree of intramural extension is uncertain, an MRI (Fig. 28.31A), hysterosalpingogram (Fig. 28.31B) or saline infusion sonogram (Fig. 28.31C) may be performed to help plan the surgical approach and to estimate the likelihood of complete excision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree