EPIDEMIOLOGY
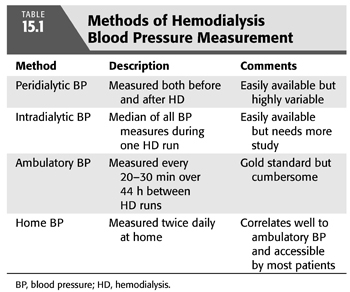
The diagnosis of hypertension is complicated in the hemodialysis (HD) patient due to the episodic nature of HD and the wide fluctuations in blood pressure (BP) that result, with BP typically highest before the HD session, lowest at the end of the HD session, and slowly rising during the interdialytic period (5). Therefore, the epidemiology of hypertension in HD can vary depending not only on the BP threshold employed but also on when and where BP is measured: in the HD clinic before or after dialysis (termed peridialytic BP) versus outside the dialysis unit using ambulatory BP monitoring (ABPM) or home BP monitoring during the interdialytic period. TABLE 15.1 summarizes the various methods of assessing BP in HD.
Epidemiology Using Peridialytic Blood Pressure Measurements
Numerous observational studies have found a high prevalence of hypertension in HD ranging from 62% to 86% based on peridialytic BP measurements; notably, these studies employed varying threshold BP values ranging from 140/90 mm Hg to 160/90 mm Hg and variously included antihypertensive drug use in their definitions of hypertension (6–9).
More recent analyses of randomized trials have found similar rates of hypertension in the HD population using peridialytic BP. The Hemodialysis (HEMO) study of the late 1990s was a landmark multicenter trial of HD dose and dialyzer flux on survival (10) that recruited clinically stable HD patients (11). An analysis of the baseline characteristics of the first 1,238 subjects randomized into the HEMO study found 72% of the cohort to be hypertensive, defined as peridialytic BP ≥140/90 mm Hg during the baseline HD session (12). This high rate of hypertension was found despite 74% of the cohort receiving antihypertensive medications, with a median of 1.0 drug per subject.
A similarly high prevalence for hypertension was found when the BP data from another large randomized trial from the late 1990s was examined in detail (13). The original study was a double-blind and placebo-controlled clinical trial of sodium ferric gluconate that enrolled 2,535 clinically stable HD patients (14). With hypertension defined as pre-HD BP >150/85 mm Hg averaged over 1 week or the use of antihypertensive medications, an analysis of baseline data found a prevalence of 86% for hypertension in this cohort (13). Within the hypertensive subjects, only 30% were controlled, while 12% were not treated pharmacologically and 58% were treated but still uncontrolled (13), which is similar to previous reports (8).
Epidemiology Using Ambulatory Blood Pressure Measurements
A recent single-center study of 369 prevalent and clinically stable HD patients employed 44-hour interdialytic ABPM and found a prevalence for hypertension at 86% using a definition of hypertension as average ambulatory BP of ≥135/85 mm Hg or antihypertensive drug use (15); this prevalence is similar to prior small studies of ABPM in HD (16). In the cohort of 369 patients, hypertension was treated with medications in 89% of patients but was controlled adequately in only 38% of patients (15).
Thus, whether using peridialytic BP measurements or 44-hour ABPM, hypertension is common in the HD population with a prevalence ranging from 70% to 85%, and a majority of these affected patients have poor control of their hypertension.
Epidemiology in Peritoneal Dialysis Patients
It has been suggested that peritoneal dialysis (PD) controls hypertension better than HD; for example, a single-center study of 56 prevalent and clinically stable PD patients found that only 9% of the cohort was hypertensivew with BP >140/90 mm Hg by standardized auscultation as compared to a hypertension prevalence of 56% in the same center’s HD unit (17). Similarly, control of BP was compared in the retrospective Peritoneal Dialysis Core Indicators Study in the mid-1990s that found among the 926 PD patients with BP data, only 35% were hypertensive with BP >150/90 mm Hg, and the cohort having an average BP of 139/80 mm Hg as compared to a contemporaneous cohort of HD patients whose average pre-HD BP was 151/79 mm Hg and post-HD BP 137/74 mm Hg (18). A larger study using United States Renal Data System (USRDS) data from the Dialysis Morbidity and Mortality Wave 2 study in the late 1990s found that from 1,034 PD patients, only 54% had systolic BP (SBP) of >140 mm Hg while on a mean of 1.6 antihypertensive medications (19), as compared to the 70% to 85% prevalence of hypertension in HD.
However, the reduced prevalence of hypertension among PD patients is not a universal finding. A prospective study of 504 prevalent and clinically stable PD patients found a prevalence of 88% for hypertension defined as BP >140/90 mm Hg or use of antihypertensive medications, and among the hypertensive patients, only 16% were adequately controlled (20). Additionally, 24-hour ABPM was performed, and of the 414 adequate examinations, hypertension was present in 69% based on BP load >30%, with load defined as the percentage of ambulatory BP readings >140/90 mm Hg during the day or >120/80 mm Hg at night. Another study utilized ABPM to compare 22 HD patients versus 24 PD patients that were well matched for major clinical characteristics and found no significant difference in either daytime or nighttime BP between the two groups (21). Thus, while some studies suggest that hypertension control is superior among PD patients versus HD patients, there is no conclusive evidence that this is the case.
 PATHOGENESIS
PATHOGENESIS
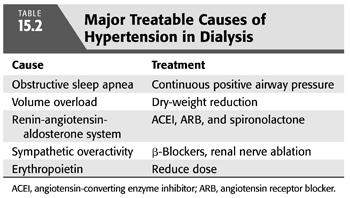
As both a cause and consequence of kidney disease, the pathogenesis of hypertension in CKD not only shares commonalities with the general hypertensive population but also has complicated causes that are unique to kidney disease and its treatment. TABLE 15.2 summarizes the major modifiable causes of hypertension in dialysis.
Risk Factors in Common with the General Population
Patients with CKD often carry a burden of preexisting primary hypertension prior to the recognition of their kidney disease. Additionally, risk factors in the general hypertensive population are similarly present in the CKD population including obesity, excessive salt intake, alcohol consumption, and physical inactivity. Two additional comorbidities contributing to hypertension are important to consider, namely, arterial stiffness and obstructive sleep apnea (OSA).
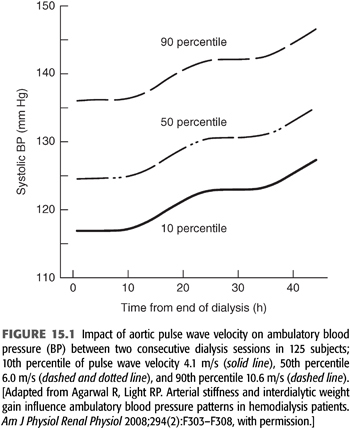
Arterial stiffness, measured as pulse wave velocity, is elevated in dialysis patients (22,23), and it has long been recognized as a risk factor for cardiovascular events in both the general hypertensive population (24) and in the dialysis population (25,26). It is in this setting that a study of 125 subjects on HD found increased pulse wave velocity to be significantly associated with increased 44-hour interdialytic ambulatory BP (27), as illustrated in FIGURE 15.1.
In the general population, OSA frequently coexists with hypertension (28–30), with hypopnea leading to hypoxemia and ultimately to sympathetic activation. OSA is strongly linked with resistant hypertension as the presence of OSA is a risk factor for resistant hypertension and the severity of OSA correlates with the severity of hypertension (31–33). Given this link, it is important to note that OSA is very common in the setting of CKD with the prevalence increasing with declining kidney function (34), culminating in a prevalence over 50% for those patients on dialysis (34,35). The association between OSA and resistant hypertension is similarly strong in ESKD as a recent cohort study of subjects with advanced CKD including 75 subjects on HD and 20 on PD found a sevenfold increased risk of resistant hypertension in those dialysis patients with severe OSA (36). Interestingly, there is a growing recognition that OSA itself is caused or exacerbated by volume overload that leads to parapharyngeal edema, which worsens at bedtime in the recumbent position, both in patients without CKD (30) and in those with ESKD (37,38).
While most studies of continuous positive airway pressure (CPAP) for OSA in the non-CKD population find significant improvement in BP, this improvement is typically modest compared to medication (39), with CPAP use yielding a 1.7 mm Hg improvement in mean 24-hour ambulatory BP (40), so similarly modest improvements would be expected with CPAP treatment in ESKD.
Classical Mechanisms of Hypertension in Dialysis: Volume Overload and the Renin-Angiotensin-Aldosterone System
The two mechanisms classically felt to be responsible for hypertension in the “renoprival” state of ESKD are volume overload and an inappropriately activated renin-angiotensin-aldosterone system (RAAS) (41). Sodium loading has long been clinically recognized as a major and essential contributor to hypertension both in those with normal kidney function (42) and in the setting of kidney disease. As the glomerular filtration rate (GFR) declines, less sodium is filtered leading to sodium retention and to an expanded extracellular fluid volume. The increased plasma volume leads to increased cardiac output and then to increased total peripheral resistance, whereby normal kidney autoregulation would lead to a pressure natriuresis and normalization of BP (43); however, this natriuresis is incomplete or absent in advanced CKD and ESKD, and the increased total peripheral resistance persists. While ultrafiltration has been recognized as an effective means of BP control in ESKD for decades since the earliest days of chronic dialysis (44), only recently has a randomized clinical trial, the Dry-Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) trial, confirmed this truism (45).
The other classically recognized contributor is an inappropriately activated RAAS (46), possibly provoked by kidney ischemia in patients with renovascular disease or by regional intrarenal ischemia due to kidney fibrosis. As expected, angiotensin-converting enzyme inhibitor (ACEI) therapy has been shown to be effective at reducing BP in dialysis patients (47,48).
Novel Mechanisms of Hypertension in Dialysis
In addition to the two classically recognized causes of hypertension in dialysis patients, several additional contributors have gained widespread acceptance including increased sympathetic nervous system activity, altered vascular endothelial function, and erythropoiesis-stimulating agents.
Sympathetic Nervous System Overactivity
Among the novel mechanisms of hypertension in advanced kidney disease, sympathetic overactivity is now widely recognized as a contributor. Increased catecholamine levels (49) and increased catecholamine sensitivity (50) in CKD were both demonstrated in the 1980s, and increased catecholamine levels have been shown to predict cardiovascular events and mortality in chronic HD patients (51). Unidentified uremic toxins were originally thought to provoke this sympathetic overactivity; however, Converse et al. (52) implicated the diseased kidneys themselves via experiments where they measured muscle sympathetic nerve activity in three groups of subjects: those on chronic HD with their native kidneys, those on chronic HD status post bilateral nephrectomy, and normal controls (52). They found increased sympathetic activity and higher BP in those chronic HD patients still with their native kidneys, but those subjects who were surgically anephric had sympathetic nerve activity and BP similar to the normal controls. Uremic toxins do not appear to be the cause of the increased kidney afferent nerve signals that increase sympathetic activity, as demonstrated by studies of patients who have had kidney transplantation and still retain their native kidneys (53), but kidney ischemia is a likely contributor (54). As further evidence, a pilot study of kidney denervation by endovascular radioablation with a before-after design performed in 12 chronic HD patients with resistant hypertension showed a significant improvement in BP (55); however, with a recent negative result from a large randomized clinical trial employing this technique in subjects without CKD, it may not be developed further (56).
Another unique contributor to sympathetic overactivity in ESKD is renalase, which was discovered only in the last 10 years (57). Renalase is a circulating monoamine oxidase enzyme that is produced primarily by the kidney that metabolizes circulating catecholamines and which is deficient in ESKD (58). As a novel mechanism contributing to hypertension in ESKD, it remains to be demonstrated via clinical trials what improvement in BP may be achieved by increasing levels of renalase in the dialysis population.
Altered Endothelial Function
Additional factors contribute to increased vasoconstriction in kidney disease, including altered endothelial cell function such that there is an imbalance in the levels of endothelial derived vasoconstrictors versus vasodilators. Nitric oxide is a potent vasodilator and is synthesized by endothelial nitric oxide synthase (eNOS) from L-arginine; however, a circulating inhibitor of eNOS has been identified in CKD patients, asymmetric dimethyl arginine (ADMA) (59). ADMA is a product of protein metabolism that is excreted into the urine by the healthy kidney, competitively inhibits eNOS, and causes local vasoconstriction on experimental infusion, and ADMA levels are elevated in ESKD (60). Elevated ADMA levels have also been shown to predict cardiovascular events and mortality in a cohort of 225 chronic HD patients (61). It remains to be demonstrated via interventional trials what improvement, if any, reductions in ADMA will make to hypertension in the dialysis population.
On the side of vasoconstriction are endothelins, which are released from the basolateral surface of endothelial cells and exert autocrine and paracrine functions that include mediating vasoconstriction via the endothelin-A receptor on vascular smooth muscle (62). Endothelin levels have been found to be elevated in various stages of CKD including ESKD (63,64). Endothelin receptor antagonists have shown efficacy for reducing BP in randomized controlled trials in primary (65) and resistant hypertension (66,67) as well as in diabetic nephropathy (68). However, some results have been mixed with divergent BP results between office and ABPM (67,68), and adverse events including edema have been frequent.
Erythropoiesis-Stimulating Agents
Conventional medications such as over-the-counter nonsteroidal anti-inflammatory drugs and decongestants can exacerbate hypertension; however, erythropoiesis-stimulating agents (ESAs) are commonly prescribed for the anemia of CKD, and resultant hypertension has been recognized since the early days of ESA use in ESKD (69). The incidence of hypertension provoked by ESA administration is associated with the ESA dose but is independent of red blood cell mass or viscosity (70,71). While the exact mechanism of how ESA use causes hypertension is unknown, the current evidence suggests that it is mediated via vasoconstrictor effects, likely through increased levels of endothelin-1 or increased vasoconstrictive response to that peptide (72–74).
Up to 30% of dialysis patients develop hypertension or require an adjustment in antihypertensive medications with ESA use (75,76), while the rise in BP with ESA use typically ranges from 5 to 8 mm Hg in SBP and 4 to 6 mm Hg in diastolic blood pressure (DBP) (77). The rise in BP with ESA administration is more likely in those with baseline hypertension (78) or a family history of hypertension (79). There is unfortunately a dearth of evidence to guide the prevention of ESA-induced hypertension but recommended strategies include changing to subcutaneous administration, reducing the goal hemoglobin level in those who are unresponsive to ESA therapy, minimizing the ESA dose by starting low and increasing slowly, and avoiding ESA use entirely (80).
 DIAGNOSIS
DIAGNOSIS
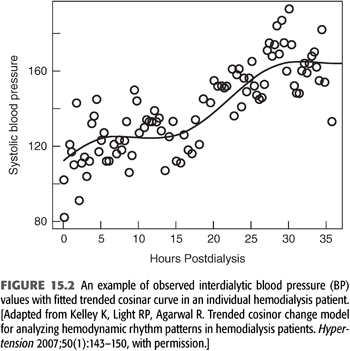
ABPM is the accepted gold standard for diagnosing hypertension in the general population and in the ESKD population on dialysis (81–84). The use of ABPM permits the diagnosis of nocturnal hypertension, which is common in the ESKD population (85,86), and it is also superior to peridialytic BP measurements for correlating to end organ damage manifest as left ventricular hypertrophy (LVH) (87) and for predicting the outcome of mortality (88). The proper ABPM technique includes employing a validated monitor (89) to measure BP every 20 minutes during the day from 6 AM to 10 PM and then every 30 minutes at night from 10 PM to 6 AM (90). This prolonged interval of measurement permits observation of the full change in BP during the interdialytic period, where SBP increases an average of 2.5 mm Hg every 10 hours (5,27), as depicted in FIGURE 15.2.
While ABPM is the gold standard method for diagnosing hypertension, it is also cumbersome to use especially over 44 continuous hours so it remains a research technique in ESKD. More convenient methods of routinely measuring BP and diagnosing hypertension must be employed in the dialysis population. Since BP changes both during the HD session and during the interdialytic interval, there remains uncertainty about how best to diagnose and manage hypertension in the HD population, and this uncertainty contributes to both under treatment (16,91) and to over treatment of hypertension (92). Further complicating matters, HD patients have significant seasonal BP variability with lower BP during the summer and higher during the winter (93), potentially due to temperature mediated vasodilation or sweat induced volume losses.
Classically, the peridialytic BP measures taken before and after an HD session have been used to diagnose and manage hypertension, and while there are no randomized clinical trials to guide goal BP recommendations in ESKD, long-standing professional guidelines have employed peridialytic BP values. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines recommend to target pre-HD BP <140/90 mm Hg and post-HD BP <130/80 mm Hg (94). Unfortunately, making treatment decisions based on peridialytic BP can be associated with adverse outcomes, as shown by a study of 11 HD units in London, England, that found those HD units with more patients reaching the post-HD BP goal had significantly more episodes of symptomatic hypotension requiring saline infusion (95). Peridialytic BP is highly variable such that the variability within a given patient over time is similar to the variability between patients (96), possibly due in part to these measurements often being made without attention to technique (97). While peridialytic BP does have a statistically significant relationship to gold standard interdialytic ABPM (98), a meta-analysis of 18 studies comparing peridialytic BP and interdialytic ABPM found very wide limits of agreement between the techniques such that peridialytic BP provides a very imprecise estimate of interdialytic BP (99). In the meta-analysis, the limits of agreement between pre-HD SBP and interdialytic ambulatory SBP ranged from +41.7 mm Hg to −25.2 mm Hg and the limits of agreement for post-HD SBP were similarly wide, which illustrates the reduced clinical utility of a diagnosis of hypertension or normotension based on peridialytic BP for an individual HD patient (99).
A readily available alternative to peridialytic BP is to use all the BP measurements made during a single midweek HD session to calculate a median BP, which is easier to calculate at the bedside compared to mean BP. A study of 150 chronic HD patients found that median intradialytic BP had the best reproducibility and was superior to either pre- or post-HD BP or their average for predicting 44-hour interdialytic ambulatory BP (100). In this study, median intradialytic SBP >140 mm Hg during a midweek HD session had an 80% sensitivity and specificity for diagnosing hypertension by gold standard ABPM (100). Median intradialytic BP has also been shown to change in response to interventional reduction in dry weight to reduce BP (101), adding further support for the clinical usefulness of this easily calculated measure.
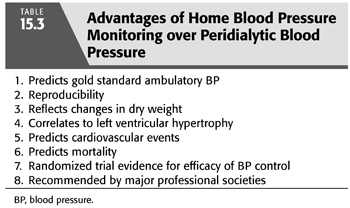
After peridialytic BP and median intradialytic BP, home BP monitoring is the third alternative to the clinically inconvenient gold standard of ABPM. Home BP monitoring is the recommended method of both the American Heart Association (102) and the European Society of Hypertension (103) for the routine diagnosis and management of BP in the general hypertension population and its use is feasible and practical both in patients with CKD and ESKD (104,105). Importantly, home BP monitoring is superior to peridialytic BP measurements by every methodologic and clinical standard. This includes superior correlation with gold standard ABPM (106), week-to-week reproducibility (107), the ability to reflect BP changes from interventional probing of dry weight (107), correlation to the end-organ damage of LVH (87,108), and predicting outcomes including cardiovascular events (109) and mortality (109,110). TABLE 15.3 summarizes the advantages of home BP monitoring.
Two small randomized trials have found home BP monitoring to be beneficial versus usual care for management of BP in HD patients. The first randomized 34 patients to home BP monitoring plus usual care versus usual care only over 12 weeks and found that the home BP group had significantly lower BP at the end of the study (111). A subsequent trial of 65 HD patients randomized participants to usual hypertension care based on pre-HD BP versus open label monthly home BP monitoring for 6 months (112). At the end of the trial, the home BP monitoring group had a significant reduction in ambulatory SBP both from baseline and versus the usual care group, which had no change in ambulatory SBP from baseline. However, the primary endpoint of reduction in echocardiographic LVH was no different between groups, possibly due to lack of power and variability in the timing of the echocardiograms relative to the HD schedule (112).
As with ABPM, home BP increases between HD sessions, at an average rate of 4 mm Hg every 10 hours (113), so it is important to adequately sample home BP at spaced intervals between HD sessions. Randomized trials have used protocols of home BP monitoring performed twice daily over 4 to 7 days once per month (112,114), and we recommend a similar regimen for routine clinical use and decision making. Measurements performed more than once per month may be needed in more unstable patients including those recently discharged from the hospital. TABLE 15.4 summarizes our suggested method of home BP monitoring.

There are no randomized trials comparing goal BP levels in the dialysis population using any of the available BP methods including peridialytic BP, intradialytic BP, home BP, or interdialytic ambulatory BP. However, the American Heart Association defines hypertension as home BP >135/85 mm Hg on average for the general population (102), so we advocate for an interdialytic home BP target of ≤140/90 mm Hg as a reasonable goal (80), which was the target used in a recent large randomized trial of BP control in HD (114). Notably, the results of the randomized controlled Systolic Blood Pressure Intervention Trial (SPRINT) were published in November 2015 showing improved cardiovascular outcomes in over 9,000 subjects randomized to office SBP target <120 mm Hg versus a conventional target of <140 mm Hg (115). While subjects with CKD were included in the trial, those with estimated GFR <20 mL/min/1.73 m2 or ESKD were excluded so no direct conclusions can be drawn from the trial regarding the management of hypertension in the dialysis population.
Intradialytic Hypertension
The focus of diagnosis and treatment of hypertension in chronic HD is on interdialytic BP between HD sessions, but the case of intradialytic hypertension merits special mention. BP normally declines during the HD, but approximately 5% to 15% of chronic HD patients have a paradoxical rise in BP during the HD session (116). Intradialytic hypertension has been described variously, and there currently is no universally accepted definition. Definitions have included (a) a change in SBP or mean arterial pressure from pre-HD to post-HD greater than a given threshold ranging from >0 mm Hg to ≥10 mm Hg change (117,118), (b) a positive slope after regression of all intradialytic SBP values (119), or (c) BP increase during or immediately following HD resulting in post-HD BP >130/80 mm Hg (116).
Intradialytic hypertension has recently been recognized to be associated with worse outcomes. Inrig et al. (120) performed a secondary analysis of a randomized clinical trial including 443 prevalent HD subjects and found intradialytic hypertension to be significantly associated with greater mortality at 6 months (117). Similarly, in a subsequent observational study of a cohort of 1,748 incident HD patients, Inrig et al. (120) found 2-year survival to be significantly decreased for each 10 mm Hg increase in SBP from pre-HD to post-HD BP; however, this relationship was limited to those patients whose pre-HD SBP was <120 mm Hg. Most recently, a prospective cohort study of 115 prevalent HD patients found an average pre-HD to post-HD rise in SBP of >5 mm Hg to significantly predict both all-cause and cardiovascular mortality (121).
Intradialytic hypertension has been associated with interdialytic hypertension as measured by 44-hour ABPM (118,119), so it is not surprising that the same mechanisms implicated in causing interdialytic hypertension between HD sessions have also been implicated in causing intradialytic hypertension during the HD session (116), but the preponderance of evidence currently points to volume overload and endothelial dysfunction. Markers of volume overload such as increased cardiothoracic ratio has been associated with intradialytic hypertension (121), but most importantly, interventional trials have shown volume removal through dry-weight reduction improves intradialytic hypertension. An early study from the mid-1990s included seven patients with intradialytic hypertension and found them all to have marked cardiac dilation and to be very hypertensive with mean pre-HD BP 172/99 mm Hg despite medications (122). Dry weight was reduced in all subjects with an average weight loss of 6.7 kg that was associated with an improvement in pre-HD BP by 46/21 mm Hg despite discontinuation of all BP medications. More recently, a secondary analysis of the DRIP trial (45) regressed the intradialytic BP values for the 150 trial subjects and found that the quintile of subjects with the greatest reduction in dry weight, more than 0.94 kg reduction after the first 4 weeks of the trial, also had the most positive BP slope at baseline as they were the only quintile with intradialytic hypertension by this definition (119). Importantly, after subsequent lowering of dry weight, this same quintile had reduction in BP slopes at the finish of the trial, meaning their intradialytic hypertension had resolved such that their BP slopes were similar to the other subjects. Thus, intradialytic hypertension appears to be a marker of volume overload that is amenable to dry-weight reduction.
Endothelial dysfunction has also been identified as an important mediator as there is evidence both for a rise in endothelin-1 levels (123) and a decrease in nitric oxide during HD (124) in patients with intradialytic hypertension. The contribution of endothelial function was investigated in 25 HD patients recruited in an 8-week pilot study with a before-after design using carvedilol (125), which has been shown to block endothelin-1 release in vitro (126). Subjects were given carvedilol up to a dose of 50 mg twice a day, and while endothelin-1 levels were unchanged on carvedilol, flow-mediated dilation significantly improved (125). Of clinical importance, the frequency of intradialytic hypertension was significantly reduced from 77% of HD sessions down to 28% of sessions and average 44-hour interdialytic ambulatory SBP was also reduced from 155 mm Hg to 148 mm Hg with carvedilol treatment.
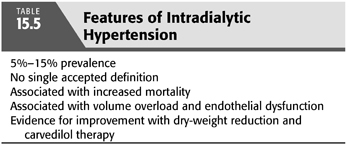
Thus, based on the available evidence, a renewed focus on addressing volume overload should be a priority for those patients with a paradoxical rise in BP on HD, and specifically targeting endothelial dysfunction with agents such as carvedilol can also be considered. The features of intradialytic hypertension are summarized in TABLE 15.5.
 PROGNOSIS
PROGNOSIS
Despite a strong and direct relationship between hypertension and both cardiovascular and all-cause mortality (127) and copious evidence of benefit for treatment of hypertension in the nondialysis population (128), the relationship between BP and outcomes in dialysis patients remains a topic of controversy (129,130). A variety of studies have found an association between peridialytic hypertension and strokes (131), heart failure (132), arrhythmias (133), cardiovascular events (134), and all-cause mortality (135). However, other studies suggest that peridialytic hypertension is protective and lower BP is associated with worse mortality (6,136–138), and the risk of normotensive BP is magnified when BP is considered as a time dependent covariate (136,138). This paradoxical relationship between BP and mortality has been termed the reverse epidemiology of hypertension (130) and has raised concern that treatment of hypertension may be harmful (139).
When examining the prognostic value of hypertension in dialysis, it is important to additionally consider the severity of comorbid illness as well as dialysis vintage. These interactions are illustrated by a retrospective cohort study of 2,770 prevalent PD patients where a fully adjusted analysis found higher SBP, DBP, mean arterial pressure, and pulse pressure, all to be associated with decreased mortality during the first year on dialysis (140). However, higher SBP and pulse pressure were associated with increased mortality for those patients on dialysis ≥6 years. Similar findings were shown in a cohort of 16,959 HD patients where SBP <120 mm Hg was associated with increased mortality within the first 2 years of starting dialysis, but SBP >150 mm Hg was associated with increased mortality among those that survived at least 3 years (141). These findings suggest lower BP may be an indicator of more severe illness in those patients new to dialysis who are likely to have advanced chronic but unstable systemic comorbidities that recently culminated in ESKD, whereas the survivors that have been on dialysis for at least 3 to 6 years have a more normal relationship between hypertension and outcomes because they are less acutely ill. This explanation is further bolstered by the subgroup in the PD cohort who were listed for transplant within 6 months of starting dialysis, as in this healthier subgroup higher SBP, DBP, mean arterial pressure, and pulse pressure were not associated with improved mortality during the first year of dialysis (140).
The technique of BP measurement also contributes to the controversial relationship between BP and outcomes as the reverse epidemiology of hypertension is primarily a phenomenon of peridialytic BP values. Ambulatory BP, however, has a strong relationship with mortality on HD, first demonstrated by Amar et al. (142) in a study of 57 HD patients. Alborzi et al. (110) have confirmed the relationship between ambulatory BP and mortality in a cohort of 150 HD patients, and in the same cohort, they also demonstrated home BP to have a similarly strong relationship with mortality. In an expanded cohort of 326 HD patients followed for a mean of 32 months, Agarwal (88) subsequently has shown increased mortality at the extremes of ambulatory and home BP and that mortality was best at a home SBP 120 to 130 mm Hg and ambulatory SBP 110 to 120 mm Hg, while peridialytic BP had no relationship with mortality in this cohort. Most recently, an analysis of the Chronic Renal Insufficiency Cohort (CRIC) study compared pre-HD SBP and out of HD unit SBP for prediction of mortality in the 403 subjects who started HD since the start of the study (143). There were 98 deaths over a mean follow-up of 2.7 years and pre-HD SBP showed a U-shaped relationship to mortality consistent with reverse epidemiology of hypertension. However, in the 326 subjects who had BP checked out of the HD unit in a standardized manner during a research visit, there was a significant and direct linear relationship between BP and mortality with hazard ratio 1.26 for every 10 mm Hg rise in SBP, which further emphasizes the importance of BP measurement technique when considering prognosis (143).
Thus, while there is concern for reverse epidemiology of hypertension when analyzing peridialytic BP, which would suggest that lowering BP would be harmful in HD patients, the evidence from ambulatory and home BP studies do not support those conclusions. Additionally and significantly, the evidence from two meta-analyses of randomized clinical trials of antihypertensive medication use in HD found cardiovascular benefit rather than harm with active treatment (144,145), so based on the available evidence, we strongly recommend to actively diagnose and treat hypertension in dialysis patients.
 TREATMENT
TREATMENT
Hypertension is common and poorly controlled in dialysis, and it often requires a combined approach to achieve adequate control of BP. The cornerstone of therapy is nonpharmacologic control of volume overload, but pharmacology therapy is frequently necessary, and invasive treatments have a long and recurrent history for the management of hypertension in dialysis patients.
Volume Control
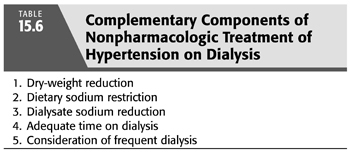
The focus of nonpharmacologic treatment of hypertension on dialysis is to treat volume overload through complementary strategies, both to reduce sodium intake by dietary sodium restriction and individualization of the dialysate sodium while also augmenting sodium removal by dry-weight reduction, providing adequate time on dialysis, and considering frequent dialysis. TABLE 15.6 summarizes the nonpharmacologic treatment of hypertension in dialysis. The archetype for this multipronged management of hypertension on dialysis is reported by Charra (146) from Tassin, France, where patients are dialyzed for extended hours on a low-sodium dialysate, and low-sodium diet is emphasized to such a degree that low-sodium bread is provided to the patients. They report excellent control of BP despite antihypertensive medication use at only 1% to 2% (147), as well as low mortality with a 5-year survival rate reported at 87% (148), which is more than twice the current reported 5-year survival rate in the United States (2). More recently, a trial of low-sodium diet and dry-weight reduction in 19 hypertensive HD patients with a before-after design found this combined strategy reduced echocardiographic LVH (149). Similar results have been reported in PD from a single center, where all 47 of the center’s hypertensive patients had their antihypertensive medications withdrawn and BP was subsequently successfully controlled in 37 patients with a combination of strict low-sodium diet and added ultrafiltration (150). A consistent feature of all these examples is the multifaceted approach to volume control requiring not only reduction in dry weight but also strict attention to reducing dietary and dialysate sodium as well as maximizing the probability of successful dry-weight reduction by providing adequate time on dialysis.
Dry-Weight Reduction
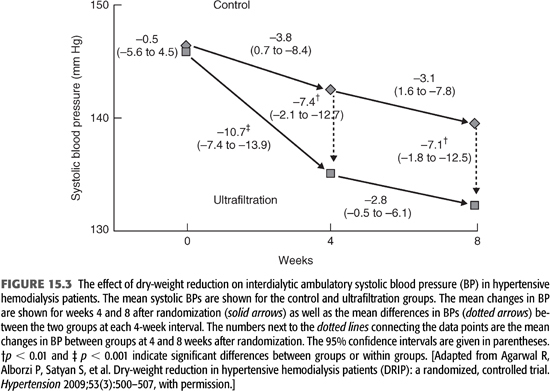
Malignant hypertension was common in ESKD prior to the advent of chronic HD, and since those earliest days, ultrafiltration has been recognized as an effective means of BP control in ESKD (44), including for the very first chronic HD patient in the United States, Clyde Shields, under the care of Dr. Belding Scribner (151). Only recently has the randomized controlled DRIP trial of dry-weight reduction definitively confirmed those original observations (45). The DRIP trial recruited 150 chronic and stable HD patients with hypertension confirmed by 44-hour interdialytic ABPM despite being on an average of 2.6 antihypertensive medications who were then randomized in a two-to-one ratio to intervention versus usual care for the 8-week trial. All subjects had their antihypertensive medications and their prescribed time on HD kept stable, and all were visited by a study physician on each HD session during the trial. The intervention group received progressive reduction in dry weight by at least 0.2 kg each HD session until they had symptoms of hypovolemia. Compared to the control group at 8 weeks, the intervention group had 1 kg of weight reduction and average 44-hour interdialytic ambulatory BP improved by 6.6/3.3 mm Hg (45), as illustrated in FIGURE 15.3. Notably, by design, those in the intervention group necessarily had to have symptoms of hypovolemia before dry-weight reduction was stopped, but despite this requirement, there was no change in any domain of the Kidney Disease Quality of Life questionnaire during the trial. Additionally, it is important to note that the DRIP trial improved BP with additional ultrafiltration without extending time on HD, which is a finding supported by older observational studies that suggest BP can be improved without extending time on HD provided volume overload is successfully reduced (152).
Management of Dry Weight
Unfortunately, there is no single universally accepted definition of dry weight. We suggest a reasonable definition of dry weight to be the lowest tolerated post-HD weight achieved via gradual change in post-HD weight at which there are minimal signs or symptoms of either hypovolemia or hypervolemia (153). Thus, achieving and maintaining an adequately low dry weight is a hands-on and iterative process that requires attention to details beyond only the prescribed dry weight (154), including adherence to a low-sodium diet, minimization of dialysate sodium content, providing adequate time on HD, and consideration of more frequent dialysis.
When deciding whether to adjust the dry weight prescription, the first step is the assessment for volume overload. Unfortunately, while the routine clinical exam performs well at detecting acute or massive volume overload, it performs poorly for detecting subtle and chronic volume overload (153). This is illustrated by a cross-sectional study of 150 chronic HD patients that found the presence of pedal edema to have no correlation with putative objective markers of volume overload including brain natriuretic peptide, echocardiographic inferior vena cava diameter, or relative plasma volume slope (155). As another example, it is important to consider that all the hypertensive subjects of the DRIP trial were at their clinical dry weight as determined by their primary nephrologist to start the trial, yet the subjects of the intervention group had their dry weight successfully reduced, which resulted in a clinically significant improvement in 44-hour ambulatory BP (45). This further highlights the difficulty in detecting subtle volume overload that if ameliorated by means of dry-weight reduction will result in improved BP.
A number of experimental objective measures of volume status have been studied including natriuretic peptides, inferior vena cava diameter, relative plasma volume monitoring, and bioelectrical impedance analysis (156). The latter two have the most supporting evidence with a secondary analysis of the DRIP trial showing that baseline relative plasma volume monitoring identified the most volume overloaded subjects, who subsequently had the largest average reduction in weight at 1.5 kg and the largest improvement in ambulatory SBP at 12.6 mm Hg (157). Most recently, a randomized trial of bioelectrical impedance analysis to guide dry weight management in a cohort of largely normotensive HD subjects found a significant improvement in LVH as well as improvement in peridialytic BP despite reductions in antihypertensive drug use for the intervention group (158).
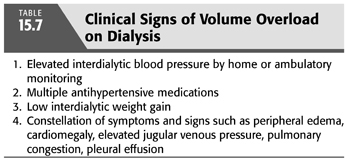
However, these objective measures of volume status remain investigational and remain to be adequately validated. Therefore, the onus is on the treating nephrologist to have a high index of suspicion for occult volume overload. Signs that should prompt consideration for reduction in dry weight include uncontrolled hypertension, especially in those patients who are on multiple medications such as in the DRIP trial where subjects were on an average of 2.6 antihypertensive drugs at baseline (45). Numerous studies have shown that greater antihypertensive drug use is associated with worse control of hypertension (15,159), which is plausibly due to inadequately addressed volume overload which could be improved with reduction in dry weight. TABLE 15.7 summarizes the clinical signs of volume overload.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


