Hepatocellular Cancer: Clinical Management
Alan P. Venook
Ronnie T. P. Poon
Derrick Wong
Theodore Lawrence
Primary hepatocellular carcinoma (HCC) is among the most common cancers of solid organs. Worldwide, this cancer occurs in more than 1 million individuals yearly and results in an almost equal number of deaths. The reason that most patients die is partly because of the insidious nature of the growth of this cancer, which usually does not present with clinical findings until late in the course of disease. Furthermore, this cancer occurs most commonly in patients with cirrhosis, which makes surgical and ablative treatments difficult. Nevertheless, the past decade has seen significant improvements in the surgical, ablative, chemotherapeutic, and radiotherapeutic options for treatment of HCC. Recent advances in therapies are summarized, with an emphasis on current standard practice and ongoing controversies.
Clinical Presentation
Most cases of HCC present at an advanced stage, well beyond the reach of curative therapies. This is due to the relatively asymptomatic nature of small HCCs. Until such time as significant liver function compromise occurs, either because of tumor invasion into major vasculature (1,2,3) or significant replacement of the liver by tumor, the few symptoms that occur are often vague and nonspecific. When tumors are large, local symptoms are common and usually include a dull, right upper quadrant ache that is often referred to the shoulder. The liver, tumor, or both are often palpable as hard and irregular. General symptoms, including anorexia, nausea, lethargy, fever, and weight loss, may be due to both the malignancy and the cirrhosis. The triad of right upper quadrant pain, mass, and weight loss is a common presentation (4,5,6).
Symptoms of hepatic decompensation are a potential presentation of HCC and include encephalopathy, jaundice (4,7,8,9), or bleeding from esophageal varices (3). In fact, jaundice occurs in up to one-half of all patients. When patients present with jaundice, distinguishing jaundice due to hepatic parenchymal insufficiency (4,7,8,9) from that due to biliary obstruction (4,10,11,12,13,14,15) is extremely important. Jaundice due to liver failure has no therapy, and survival is on the order of weeks, whereas that due to biliary obstruction can usually be palliated and may even be treated with curative intent (12,13,16,17,18,19,20).
Rare cases of HCC (<5%) can present with paraneoplastic syndromes due to hormonal or immune effects of the tumors (21). The most important of these are hypoglycemia and hypercalcemia. When hypercalcemia is present, it must be distinguished from the hypercalcemia seen with widespread bony metastases. A bone scan will distinguish these two clinical scenarios, which have different prognostic implications.
A rare but devastating presentation of HCC is spontaneous rupture, which occurs in approximately 2% of patients (22,23,24,25,26,27). Patients present with acute abdominal and hemodynamic instability. An abdominal scan demonstrating hepatic mass and hemoperitoneum confirms the diagnosis (26,28,29). Patients with a history of chronic hepatitis or cirrhosis presenting with an abdominal catastrophe should be suspected of having a ruptured hepatoma. Diagnosis by cross-sectional scanning or by paracentesis may lead to lifesaving angiography and embolization, which are now preferred to exploratory laparotomy.
Definitive diagnosis of HCC can usually be established noninvasively using a combination of history, physical examination, imaging, and blood tests. In a patient with chronic hepatitis or cirrhosis, the findings of a liver mass and a serum alpha-fetoprotein (AFP) level of >500 ng/dL are diagnostic. In a patient with a potentially resectable liver mass and a nondiagnostic AFP level, most surgeons would proceed to potentially curative resection if imaging is suggestive of cancer and no other primary sites of tumors that may have metastasized to the liver are found. Most would avoid a percutaneous liver biopsy, which may be complicated by hemorrhage, tumor rupture, tumor spillage, and seeding of the needle tract (30).
In patients with nondiagnostic AFP levels who are not candidates for curative therapy, fine-needle aspiration for cytologic evaluation is performed (31) if patients are candidates for palliative therapy. Patients who are not candidates for palliative therapy do not need to undergo biopsy.
Potentially Curative Treatments
A wide variety of methods are used for treatment of HCC (Table 33.1). Of these, the only therapies with curative potential are partial hepatectomy and total hepatectomy with liver transplantation. Partial hepatectomy has the greatest applicability and is summarized first.
Partial Hepatectomy
Partial hepatectomy is the most common procedure performed for HCC with curative intent. In the United States, a substantial number of patients with HCC have no associated cirrhosis (32). In a noncirrhotic liver, routine recovery can be expected even after resection of more than two-thirds of functional parenchyma because of the remarkable regenerative capacity of the liver (33). At most major centers, partial hepatectomy in patients without cirrhosis has been refined to allow operative mortalities well below 5% (32,34,35,36,37,38,39,40) (Table 33.2). Patients generally stay <10 days in hospital and return to normal, functional lifestyles. Such resections are associated with a 5-year survival of one-third of patients (Table 33.3) (32,37,39,40,41,42,43,44,45,46,47,48,49,50,51,52).
Thus, when HCC occurs in patients without cirrhosis, partial hepatectomy is justified both by the low risk and by the potential for long-term survival and cure.
Thus, when HCC occurs in patients without cirrhosis, partial hepatectomy is justified both by the low risk and by the potential for long-term survival and cure.
Table 33.1 Treatment Options For Hepatocellular Carcinoma | |
|---|---|
|
Partial hepatectomy in patients with cirrhosis is associated with a higher risk. Even in centers with extensive experience in liver resection, partial hepatectomy in patients with cirrhosis was, until recently, associated with a mortality of 10% or more (Table 33.2) (34,35,37,38,53,54,55). This was the major impetus for exploring total hepatectomy and liver transplantation as treatment for HCC. Nevertheless, partial hepatectomy clearly provided good long-term outcome, provided the patient survived the operation. Patients with cirrhosis who survived the surgical procedure had a 5-year survival of approximately 30% (Table 33.3) (34,38,53,55). Since the 1990s, improvements in patient selection, operative conduct, and perioperative care have significantly decreased the operative mortality associated with partial hepatectomy in patients with cirrhosis (Table 33.2). The mortality at many centers has been reduced to <5% (32,40,42,50,56,57). Some centers have reported <2% mortality in their most recent experience (58). Given the shortage of organs for transplantation, partial hepatectomy should be considered as the curative treatment of choice for eligible patients (see “Patient Selection for Partial Hepactectomy” section).
A solitary HCC with a diameter of <5 cm is regarded by some as the best candidate for resection because of the increased risk of additional nodules or vascular invasion and, consequently, incomplete resection with larger HCCs (59,60). However, it has been shown that patients with a large solitary HCC (Fig. 33.1) are suitable candidates for successful resection, and reasonable long-term survival results can be achieved (61,62). The presence of multiple tumor nodules or vascular invasion in major intrahepatic venous branches may be associated with worse prognosis. However, surgical resection is still considered the best treatment in terms of long-term survival (63,64). Bilobar HCC used to be a contraindication for resection, but a recent study suggested that patients with a predominant mass in one lobe and one or two small tumor nodules in the other lobe may benefit from combined resection of the predominant tumor and ablation or chemoembolization for the contralateral nodules (65).
Patient Selection for Partial Hepatectomy
Partial resection with curative intent can be performed for patients with disease confined to the liver, patients with adequate hepatic functional reserve, and patients with disease anatomically disposed so as to allow adequate residual liver after resection.
A most important factor influencing outcome of patients with HCC is baseline liver functional status. The majority of patients have cirrhosis of the liver. This associated cirrhosis greatly increases the risks of any therapy (66,67), but particularly those of partial hepatectomy. The cirrhotic liver is rigid and hard, and consequently difficult to handle. The accompanying portal hypertension, varices, thrombocytopenia, and coagulopathy seen in patients with cirrhosis further increase the risk of operative hemorrhage. After surgery, the portal hypertension is exaggerated and commonly results in ascites and sometimes in variceal bleeding. The cirrhotic liver may also have difficulty regenerating, which results in liver failure. Assessment of the severity of cirrhosis is therefore paramount in patient selection. Various serum measures of liver function have been suggested as useful predictors of perioperative outcome, including elevations in serum bilirubin (68) and serum alanine aminotransferase (69), low platelet count, and prolonged prothrombin time (70).
Table 33.2 Operative Mortality After Partial Hepatectomy as Related to Liver Cirrhosis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Staging for hepatocellular carcinoma has been extensively reviewed in the preceding chapter (see Chapter 32). In brief, several staging systems have been used, including the Child-Pugh classification of liver functional derangement. The Child-Pugh classification is based on the levels of serum bilirubin and serum albumin, coagulation profile, presence or absence
of ascites and encephalopathy, and nutritional status (71,72). In general, only Child-Pugh class A patients are suitable for a major hepatectomy removing three or more segments of the liver. Child-Pugh class B patients may be suitable for minor hepatectomy, whereas Child-Pugh class C cirrhosis is generally a contraindication for resection. The bilirubin and albumin levels reflect the excretory function and synthetic function of the liver, respectively. Platelet count is also important because it reflects the severity of portal hypertension. In some centers, special tests of excretory function of the liver, such as indocyanine green clearance test and galactose elimination capacity, are used to further refine the assessment of liver function (73,74). However, these specific liver function tests reflect the function of the whole liver, while the risk of postoperative liver failure depends on the liver function reserve of the remnant liver.
of ascites and encephalopathy, and nutritional status (71,72). In general, only Child-Pugh class A patients are suitable for a major hepatectomy removing three or more segments of the liver. Child-Pugh class B patients may be suitable for minor hepatectomy, whereas Child-Pugh class C cirrhosis is generally a contraindication for resection. The bilirubin and albumin levels reflect the excretory function and synthetic function of the liver, respectively. Platelet count is also important because it reflects the severity of portal hypertension. In some centers, special tests of excretory function of the liver, such as indocyanine green clearance test and galactose elimination capacity, are used to further refine the assessment of liver function (73,74). However, these specific liver function tests reflect the function of the whole liver, while the risk of postoperative liver failure depends on the liver function reserve of the remnant liver.
Table 33.3 SURVIVAL RATES AFTER LIVER RESECTION FOR HEPATOCELLULAR CARCINOMA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
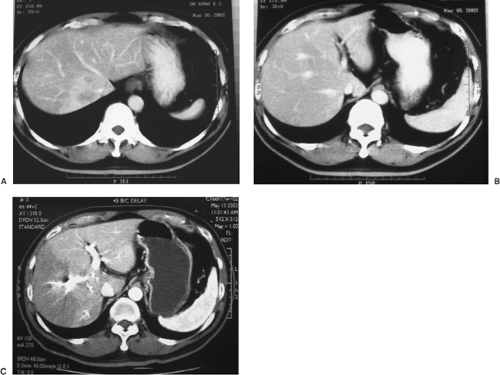
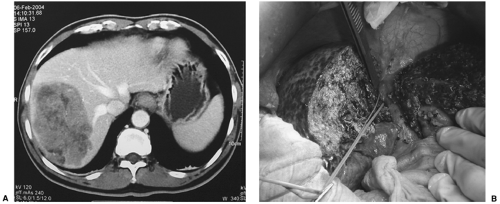 FIGURE 33.1. A cirrhotic patient with a large HCC (9 cm) (A) in the right lobe of the liver underwent right hepatectomy (B). The patient has remained diseasefree for 3 years (See also color Figure 33.1b). |
The volume of the remnant liver can be assessed by CT volumetry to provide a guideline as to the safety of major hepatic resection (75). Extended right or left hepatic resection can be performed even in the presence of cirrhosis, provided patients are carefully selected in terms of liver function reserve (62a). In patients with inadequate remnant liver volume for a right or extended right hepatectomy, preoperative portal vein embolization can be employed to induce hypertrophy of the liver remnant before resection (Fig. 33.2). The role of portal vein embolization in the cirrhotic liver remains unclear. However, one
prospective nonrandomized study suggested that preoperative right portal vein embolization induced significant hypertrophy in some patients with liver fibrosis or mild cirrhosis, and it reduced the incidence of postoperative complications compared with cases of right hepatectomy without preoperative portal vein embolization (76).
prospective nonrandomized study suggested that preoperative right portal vein embolization induced significant hypertrophy in some patients with liver fibrosis or mild cirrhosis, and it reduced the incidence of postoperative complications compared with cases of right hepatectomy without preoperative portal vein embolization (76).
A number of changes in operative conduct have combined to improve perioperative outcome. A willingness to use inflow occlusion during resection is now seen. This technique of temporarily occluding the hepatic artery and portal vein during liver resection by clamping the gastrohepatic ligament significantly decreases blood loss during hepatectomy (77). In the past, surgeons have been reluctant to use this technique in patients with cirrhosis for fear that cirrhotic parenchyma will not tolerate even transient ischemia. Recent physiological studies have proven these fears to be unfounded. That the cirrhotic liver can tolerate warm ischemia for longer than 30 minutes is now well documented (78,79). Safety has also increased with acceptance of the use of limited, nonanatomical resections in treating patients with cirrhosis. For patients with no cirrhosis, most major centers adhere to the anatomical boundaries of the various segments during liver resection for cancer because these anatomical procedures provide better tumor clearance than nonanatomical resections (80). The anatomy of the liver is shown in Chapter 32. In the cirrhotic liver, however, the smallest resection that will remove all gross tumor is generally used at most centers, with tumor clearance sacrificed for operative safety.
Patients with extrahepatic metastases are excluded from partial hepatectomy. The search for extrahepatic disease must therefore be thorough and must include the locations at greatest risk for metastases, including the lungs, peritoneum, adrenal glands, and bone. The chest should be examined by chest radiography. Computed tomography (CT) or magnetic resonance imaging (MRI) should be used to evaluate the other sites. Bone scans should be obtained if the patient has symptoms or if hypercalcemia is noted.
A helical contrast CT is the most important study to evaluate the tumor status (Fig. 33.1). In addition to assessment of tumor size and number, it provides important information regarding the relationship of the tumor to major intrahepatic portal pedicles and hepatic vein, and it also detects any major vascular invasion. HCC has a great propensity for vascular invasion and extension, and tumor thrombus in the portal vein, hepatic vein, or vena cava is therefore not unusual. Presence of tumor within the vena cava or main portal vein indicates uniformly poor prognosis. MRI is an alternative that provides similar information.
Angiography was widely used in the past to diagnose HCC, but its use is now limited to selected patients with uncertain diagnosis of HCC by CT scan because the modern three-phase CT scan can demonstrate the arterial enhancement and portal
venous washout that are typical of HCC. Some have advocated routine angiography and injection of lipiodol, a lipid that preferentially lodges within tumors, for delineation of extent of disease (81). Although angiographic techniques are highly sensitive for the presence of tumor and should be used in patients with suspected small tumors not seen by conventional cross-sectional imaging, they should not be used routinely. Helical CT or MRI suffices for staging in the majority of patients.
venous washout that are typical of HCC. Some have advocated routine angiography and injection of lipiodol, a lipid that preferentially lodges within tumors, for delineation of extent of disease (81). Although angiographic techniques are highly sensitive for the presence of tumor and should be used in patients with suspected small tumors not seen by conventional cross-sectional imaging, they should not be used routinely. Helical CT or MRI suffices for staging in the majority of patients.
Patient selection should also take into account determinants of long-term prognosis. No patient should be put at risk if improvement in outcome would not be provided by the therapy. Patients with intravascular extension of tumor have very poor outcomes. Even though tumor thrombus can be treated with liver resection and thrombus extraction, the risk of disseminated disease is extremely high, and few are cured (82). Most surgeons would therefore consider tumor thrombus involving the vena cava or main portal vein to be a relative contraindication for liver resection.
As the number of long-term survivors after partial hepatectomy has been documented to increase, many factors that in years past were believed to be contraindications to surgical resection have not been substantiated by the data. Presence of multiple lesions does not seem to preclude long-term survival (37,38). Presentation with jaundice does not preclude long-term survival after surgical resection, provided the jaundice is due to intraductal extension of tumor (35). Synchronous direct invasion of adjacent organs such as the diaphragm by HCC is potentially curable, provided the area of direct organ invasion is also resectable (83,84).
In general, those selected for partial hepatectomy are those with no cirrhosis or well-compensated cirrhosis, who have HCC confined to the liver or with resectable direct extension into an adjacent organ. Up to 80% of noncancerous liver parenchyma can be resected in patients without cirrhosis, and anatomical resections are favored in this patient population. In patients with cirrhosis, resections are performed if resection of <20% to 25% of noncancerous parenchyma is necessary for complete extirpation of tumors (37,38,85,86,87).
Current Results of Hepatic Resection
With advances in surgical techniques and perioperative management, near-zero hospital mortality rate after resection of HCC can be achieved in experienced centers (88,89). In most major centers, an operative mortality rate of <5% is the current standard. However, the morbidity rate remains high at about 30% to 40%, even in experienced centers (88,89). Life-threatening complications such as liver failure, intraabdominal hemorrhage, bile leakage, and intraabdominal sepsis are less frequent today, but wound infection and pulmonary complications remain common (88). These complications may be reduced with the use of laparoscopic approach for liver resection, which is applicable in selected patients with small HCCs in the anterior segments or left lateral segments of the liver (90).
Improvement in the long-term survival results after resection of HCC has also been observed over the past decade (90a). The 5-year survival after resection of HCC in recent large series is in the range of 35% to 50% (Table 33.3) (51,91,92,93,94,95). The recent improvement in survival may be attributed to early diagnosis of HCC and reduction in perioperative blood transfusion. Perioperative transfusion has been found to have an adverse impact on the long-term survival after resection of HCC, perhaps by an inhibitory effect on the immune system that leads to increased risk of recurrence. Hence, the surgeon can play an important role in improving the long-term prognosis of patients after resection of HCC by minimizing intraoperative blood loss and avoiding perioperative transfusion (91).
The long-term prognosis after resection of HCC has been hampered by a high incidence of postoperative recurrence due to metastatic lesions or multicentric recurrences in the liver remnant (96). Adverse tumor factors such as the presence of macroscopic or microscopic vascular invasion are the most important risk factors of recurrence, suggesting that microscopic metastasis is an important cause of recurrence. The presence of cirrhosis also adversely affects the long-term prognosis, not only because it leads to worse liver function but also because it predisposes to increased risk of multicentric recurrence. However, aggressive treatment of recurrent tumors by reresection or nonsurgical modalities, such as transarterial chemoembolization and percutaneous ablation therapy, can result in prolonged survival, even after the development of recurrent tumors (96a). Postoperative adjuvant systemic or regional chemotherapy has thus far failed to prevent recurrence in prospective clinical trials (96). A recent study has shown that apart from providing the prospect of long-term survival, hepatic resection also improves the quality of life of patients with HCC (96b). It is likely that hepatic resection will play an increasingly important role in the management of HCC with the documented safety of the operation and increasing diagnosis of small HCCs in the setting of wider screening for HCC among chronic hepatitis and cirrhotic patients.
Adjuvant Therapy
Two-thirds of patients experience recurrence after partial hepatectomy for HCC, which indicates the presence of microscopic disease undetected at the time of liver resection (97,98,99). This explains the active investigation for effective adjuvant therapy for treatment of such residual disease. Use of adjuvant therapy has been hindered by the poor efficacy of chemotherapeutic agents against this cancer cell type and the extended period needed for complete recovery of patients with cirrhosis, which makes administration of potentially toxic agents difficult. This may explain the poor results seen in adjuvant chemotherapy or chemoembolization trials. In one study in which 61 patients with resected HCC were randomly assigned to receive no further therapy or postoperative hepatic infusion of lipiodol and cisplatin with systemic epirubicin hydrochloride, the treated patients had a higher recurrence rate and a worse outcome (100). Another study in which 57 patients with resected HCC were randomly assigned to receive hepatic arterial infusional and systemic epirubicin or no adjuvant treatment found no difference in survival (101). In three different trials of chemoembolization, survival has been worse for those treated after resection (102,103,104). One study in which 49 patients with resected HCC were randomized to low-dose epirubicin and mitomycin or no adjuvant treatment did find a trend toward improvement in diseasefree survival and overall survival, but it did not reach statistical significance (105). A more recent meta-analysis of three randomized control trials, which compared resection alone versus resection followed by intraarterial and/or systemic chemotherapy in HCC patients, found no difference in diseasefree survival or overall survival (106). In cirrhotic patients, postoperative chemotherapy was associated with a worse outcome. To date, no study has shown any systemic chemotherapy or chemoembolization to improve survival after partial hepatectomy for HCC.
Four randomized trials of adjuvant therapy after resection for HCC have shown positive results. The first involved radioembolization using transarterial delivery of lipiodol tagged with iodine 131 (131I). In a prospective, randomized trial, patients who received no adjuvant therapy were compared with those who were treated with 50 mCi of transarterial 131I-lipiodol within 6 weeks of liver resection (107). The study was halted at an interim time because the preliminary data, compiled after the accrual of 43 patients, demonstrated a significant improvement in survival in the patients who received treatment. The 3-year survival rates for the treated group and
the control group were 85% and 46%, respectively. Toxicity was mild, although patients were hospitalized until the radiation had dissipated. These results are quite remarkable but require validation in a multicenter trial to confirm the feasibility of performing such radioembolization at multiple centers.
the control group were 85% and 46%, respectively. Toxicity was mild, although patients were hospitalized until the radiation had dissipated. These results are quite remarkable but require validation in a multicenter trial to confirm the feasibility of performing such radioembolization at multiple centers.
A second positive study involved the use of the retinoid derivative polyprenoic acid. This chemopreventive agent has been shown to inhibit hepatocarcinogenesis in rodents (108). In a placebo-controlled randomized trial examining patients after curative resection or percutaneous ethanol injection (PEI) for HCC, treatment with polyprenoic acid reduced tumor recurrence. Currently, this compound is not available in the United States.
A third study demonstrating an impact on HCC recurrence with adjuvant therapy involved interferon. In this small Japanese trial that randomized 30 patients who had undergone resection of their hepatitis C–associated HCC to 88 weeks of tapering interferon-alpha therapy or no adjuvant therapy, patients on the treatment arm had a significantly lower disease recurrence rate than those in the control group (P = 0.037) (109).
Finally, immunotherapy has also shown potential as an adjuvant treatment. In a randomized trial of 150 patients with resected HCC, immunotherapy via infusion of autologous lymphocytes activated with recombinant interleukin-2 and CD3 antibody was associated a longer time to recurrence and a higher 5-year diseasefree survival rate than observation (110).
The sample sizes in these adjuvant studies are small, but their positive results offer strong encouragement for further evaluation of these adjuvant therapies in well-designed multicenter randomized phase III trials to determine their efficacy and feasibility. Until such studies are undertaken and completed, postoperative adjuvant therapy following partial hepatectomy remains an investigational approach.
Liver Transplantation
Indications
In the 1980s, when liver transplantation was at the initial phase of development, advanced unresectable HCC was a common indication for transplantation. However, the results of transplantation for advanced HCC turned out to be disappointing, with a 5-year survival rate of around 20% (111). The reason for the poor survival results was the high incidence of recurrent tumors, presumably due to the presence of circulating tumor cells associated with large HCCs.
Experience of liver transplantation over the past decade has shown that tumor size and vascular invasion were the two most important factors in determining the survival results after transplantation for HCC. Recent studies have indicated that for solitary HCC <5 cm or for three or less tumor nodules each of size <3 cm, the long-term survival rate was comparable to that after resection, and the diseasefree survival rate was superior to that after resection (66,112,113,114,115). It is now well accepted that child’s C cirrhotic patients with HCC <5 cm or more than three tumor nodules each of size <3 cm and without radiologic evidence of venous invasion or distant metastasis should be treated by transplantation because hepatic resection is usually contraindicated in this group of patients with poor hepatic function (116,117). These so-called Milan criteria have become widely accepted as the selection criteria for liver transplantation for HCC (112). Recently, Yao et al. (118) suggested the expanded criteria of solitary tumor ≤6.5 cm or three or less nodules with the largest lesion ≤4.5 cm and total tumor diameter ≤8 cm for liver transplantation. Their study showed that the long-term survival after transplantation for such patients was similar to that of liver transplantation for HCCs within the Milan criteria. In that study, patients with HCC fulfilling the expanded criteria had a 5-year survival of 75.2%. Although the expanded criteria have been supported by other studies (119), there is inadequate data in the literature to validate the long-term survival results seen using the expanded criteria. Furthermore, it has to be noted that Yao’s criteria were based on pathological examination of explants rather than preoperative radiologic imaging, which often underestimates the size of the tumor compared with measurement of tumor size in the explants. Currently, most centers worldwide still adopt Milan criteria in the selection of patients for liver transplantation.
Current Results of Liver Transplantation
Table 33.4 shows the results of liver transplantation for HCC within the Milan criteria in patients with Child-Pugh class C liver cirrhosis (112,117,120,121,122,123). With the improvement in surgical techniques and better immunosuppressants to reduce the risk of graft rejection, the hospital mortality rate is <5% in major centers. The possibility of long-term survival and cure after transplantation for patients with HCC is well documented (66,112,124,125,126,127,128,129,130,131,132,133,134). The best results are seen in patients with fibrolamellar HCC. Favorable results are also seen in patients with small tumors, particularly when these are found incidentally within the explanted liver during transplantation for noncancerous indications. Overall, the 5-year survival rate is about 60% to 75%, while the 5-year diseasefree survival rate is about 60% to 70%.
The most important adverse prognostic factors of liver transplantation for HCC are adverse tumor factors such as the presence of microscopic vascular invasion and histopathological grading (123,135). Although the incidence of tumor recurrence is much lower after liver transplantation compared with partial hepatic resection, tumor recurrence is an important cause of long-term mortality after liver transplantation. Currently, there is no effective adjuvant therapy to reduce the risk of tumor recurrence. Although some authors suggested that preoperative transarterial chemoembolization may reduce the risk of tumor recurrence, the role of pretransplant chemoembolization remains uncertain because there are no data from prospective randomized trials (136).
Role of Liver Transplantation for Child’s A Cirrhotic Patients with Early Hepatocellular Carcinoma
Whether child’s A cirrhotic patients with preserved liver function and a small HCC <5 cm in diameter should be treated with transplantation or resection is a controversial issue. Some authors recommended liver transplantation for small HCC even in child’s A patients because of the superior diseasefree survival results after transplantation (113,114). Others argued that hepatic resection should be the first-line therapy for such patients because of the similar overall survival results of the two treatments and the shortage of organ donors (120,137,138). Recently, a few retrospective studies have directly compared liver resection and transplantation for these patients. Bigourdan et al. (139) reported significantly better survival results after liver transplantation than after hepatic resection. However, a closer look at the data of the study revealed that there was significant selection bias in favor of the liver transplantation group, which had smaller tumors, a higher proportion of solitary tumor, a lower incidence of vascular invasion, and a lower proportion of alcoholic cirrhosis. Shabahang et al. (140) reported similar operative mortality and survival results after resection and transplantation for HCC in child’s A cirrhotic patients, but hepatic resection was associated with faster recovery. More recently, Margarit et al. (141) reported similar survival results after resection and transplantation in child’s A cirrhotic patients,
whereas postoperative mortality was higher and hospital stay was longer in the transplantation group.
whereas postoperative mortality was higher and hospital stay was longer in the transplantation group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree