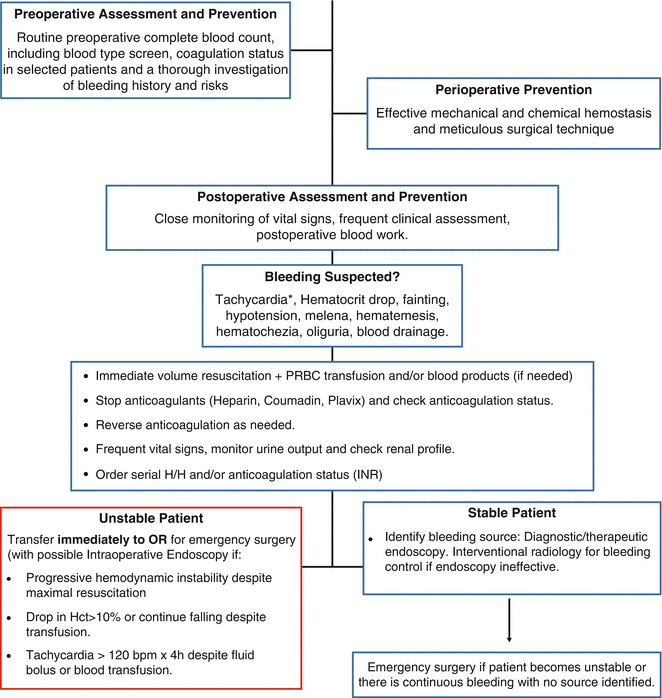
Fig. 5.1
Most common sites of bleeding after gastric bypass. © Cleveland Clinic, with permission
Extraluminal bleeding can less commonly present as late bleeding, in some cases up to several months after surgery. Some symptoms can be confusing, like sub-obstruction symptoms with weight loss, vomiting, abdominal pain, and nausea.
Special attention must be given to injuries to the abdominal aorta that can occur when entering the abdomen in any laparoscopic surgery. Intraabdominal injuries have occurred in all three techniques used to place the first trocar [30–34]. The overall risk of aortic injury with trocar placement in LRYGB is 0.043 % and up to 0.091 % when an optical trocar is used [35]. All techniques have pros and cons and the individual surgeon’s personal experience in each type of entry is of great importance to prevent this complication [35] (Fig. 5.1)
Other signs and symptoms found in both intraluminal and intraperitoneal bleeding include hypotension, dizziness, weakness or shortness of breath, hypoactive bowel movement sounds, fever, and abdominal discomfort or abdominal hematoma [1].
5.4 Diagnosis and Management
The diagnosis of hemorrhage can be challenging due to altered postsurgical gastrointestinal anatomy. There is no standard therapeutic strategy defined for diagnosis and management. Clinical presentation and timing of bleeding will dictate the most appropriate diagnostic and therapeutic strategy. In some cases, the source of bleeding can be identified based on the clinical presentation without the need of endoscopy or imaging studies. For instance, hematemesis in a gastric bypass patient strongly suggests bleeding from a proximal source such as the gastric pouch or gastrojejunostomy. Melena, on the other hand, usually comes from a bleeding source at the jejunojejunostomy or gastric remnant [3].
After suspicion of hemorrhage is established, a careful physical examination should be performed. Hematocrit/hemoglobin should be drawn and close monitoring of vital signs initiated, including heart rate, blood pressure, urine output, respiratory rate, and pulse oximetry. The systolic blood pressure may not decrease significantly until 25–40 % of blood volume is lost, so other signs of instability must be taken into consideration [36]. Frequent clinical assessments should be made. Attention should be paid to symptoms such as: pain level, shortness of breath and lethargy and objective symptoms such as tachycardia, changed mental status, decreased urine output, decreased/increased respiration, abdominal distension, peritoneal signs, color and volume drain output, bleeding at port sites or bruising, bloody/black stools, bloody vomiting, signs of intestinal obstruction (from occluding blood clots), and jaundice (from absorbing blood or hemophilia).
In the hemodynamically stable patient: Immediate resuscitation fluids (crystalloid or PRBC) and close monitoring (transfer to ICU if judged necessary). The recommendations and suggestions of the 2015 practice guidelines for perioperative blood management of the American Society of Anesthesiologists are as follows [37]:
The determination whether hemoglobin concentrations between 6 and 10 g/dL justify or require red blood cell transfusions should be based on potential ongoing bleeding (rate and magnitude), intravascular volume status, signs of organ ischemia, and adequacy of cardiopulmonary reserve.
Red blood cells should be administered unit-by-unit, when possible, with interval reevaluation. The maximal surgical blood order schedule should be used, in accordance with your institutional policy.
Anticoagulants should be reversed, if previously used. Urgent reversal for warfarin requires Prothrombin Complex Concentrate (PCC) while vitamin K may be used for non-urgent reversal.
Treatment of excessive bleeding: start by obtaining a full platelet count and a test of platelet function, if available, in patients with suspected drug induced platelet dysfunction. Platelet transfusion may be indicated despite an apparently adequate platelet count or, in the absence of a platelet count, if there is known or suspected platelet dysfunction and in surgical or obstetric patients. Platelet transfusion is rarely indicated when platelet count is known to be greater than 100 × 109/l and is usually indicated when the count is less than 50 × 109/l in the presence of excessive bleeding.
Obtain coagulation tests (PT/INR and aPTT) before transfusion of fresh frozen plasma (FFP)—if results are normal, FFP should not be used. FFP may be indicated for excessive microvascular bleeding in the presence of an INR > 2.0 in the absence of heparin, in urgent reversal of warfarin when no PCCs are available, for correction of excessive microvascular bleeding secondary to coagulation factor deficiency in patients transfused with more than one blood volume (approximately 70 mL/kg) and when PT or INR and aPTT cannot be obtained in a timely fashion.
Assess fibrinogen levels before the administration of cryoprecipitate, if possible. Such evaluation is indicated when a test of fibrinogen activity indicates fibrinolysis, when the fibrinogen concentration is less than 80–100 mg/dL in the presence of excessive bleeding, as an adjunct in massively transfused patients when fibrinogen concentrations cannot be measured in a timely fashion, and for patients with congenital fibrinogen deficiencies. Whenever possible, decisions regarding patients with congenital fibrinogen deficiencies should be made in consultation with the patient’s hematologist
Desmopressin and topical hemostatics such as fibrin glue or thrombin gel can be used. Consider the use of antifibrinolytics if fibrinolysis is documented or suspected and if these agents are not already being used.
PCCs may be used in patients with excessive bleeding and increased INR.
Consider recombinant activated factor VII when traditional options for treating excessive bleeding have been exhausted.
A formula that helps calculate the drop in hematocrit with the use of the estimated blood loss is available [38]:
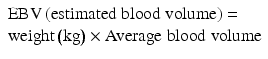 EBV for Adult Men is 75 mL/kg and for Adult Women 65 mL/kg
EBV for Adult Men is 75 mL/kg and for Adult Women 65 mL/kg
![$$ \begin{array}{l}\mathrm{Allowable}\;\mathrm{Blood}\;\mathrm{Loss}=\left[\mathrm{E}\mathrm{B}\mathrm{V}\times \left({\mathrm{H}}_{\mathrm{i}}-{\mathrm{H}}_{\mathrm{f}}\right)\right]/{\mathrm{H}}_{\mathrm{i}} \\ {}\left({\mathrm{H}}_{\mathrm{i}} = \mathrm{initial}\ \mathrm{hematocrit}\ \mathrm{and}\ {\mathrm{H}}_{\mathrm{f}} = \mathrm{final}\ \mathrm{lowest}\ \mathrm{hematocrit}\ \mathrm{accepted}\right).\end{array} $$](/wp-content/uploads/2017/04/A318404_1_En_5_Chapter_Equb.gif) If the patient remains hemodynamically stable, endoscopic inspection of the gastrojejunostomy should be considered. Thermal coagulation or epinephrine injections via therapeutic endoscopy have been successful in the management of bleeding at the gastrojejunostomy and jejunojejunostomy [3, 39, 40]. This procedure is effective for both late bleeding and early bleeding in patients who are hemodynamically stable.
If the patient remains hemodynamically stable, endoscopic inspection of the gastrojejunostomy should be considered. Thermal coagulation or epinephrine injections via therapeutic endoscopy have been successful in the management of bleeding at the gastrojejunostomy and jejunojejunostomy [3, 39, 40]. This procedure is effective for both late bleeding and early bleeding in patients who are hemodynamically stable.
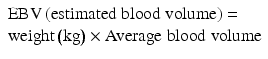
![$$ \begin{array}{l}\mathrm{Allowable}\;\mathrm{Blood}\;\mathrm{Loss}=\left[\mathrm{E}\mathrm{B}\mathrm{V}\times \left({\mathrm{H}}_{\mathrm{i}}-{\mathrm{H}}_{\mathrm{f}}\right)\right]/{\mathrm{H}}_{\mathrm{i}} \\ {}\left({\mathrm{H}}_{\mathrm{i}} = \mathrm{initial}\ \mathrm{hematocrit}\ \mathrm{and}\ {\mathrm{H}}_{\mathrm{f}} = \mathrm{final}\ \mathrm{lowest}\ \mathrm{hematocrit}\ \mathrm{accepted}\right).\end{array} $$](/wp-content/uploads/2017/04/A318404_1_En_5_Chapter_Equb.gif)
EGD for early bleeding should optimally be performed under general anesthesia, in the operating room and with endotracheal intubation [10, 41]. Endoscopy has shown to control acute bleeding from the gastrojejunal anastomosis; endoscopic management of jejunojejunostomy hemorrhage has also been described [39, 40]. When the source of the gastrointestinal hemorrhage in a gastric bypass patient is not visualized endoscopically, the gastric remnant or the duodenum should be suspected [27]. The bypassed stomach of the RYGB patient is inaccessible by conventional endoscopy, so an alternative method of access must be utilized. These approaches include laparoscopic transgastric endoscopy in which a laparoscopic trocar is surgically placed into the gastric remnant and serves as a conduit for passage of the flexible endoscope [10], percutaneous endoscopic gastrostomy [42] and retrograde double balloon endoscopy [43, 44]. A more complex method of gastric remnant access involves the use of a double-balloon endoscope to achieve retrograde endoscopy. This technique is particularly technically challenging and requires a specially trained endoscopist to perform [45, 46].
Hemorrhage in an unstable patient: Unstable vital signs, such as hypotension, persistent severe tachycardia, and drop in hematocrit of 10 % or continuous dropping after transfusion indicates the need for urgent surgical intervention. Also, frank hematemesis or bright red blood per rectum within the first 6 h after surgery with a decline in hematocrit indicates active bleeding which will most likely require surgical intervention [2]. The objective of the reoperation is to identify the bleeding source, decompress the lumen from blood and blood clots, and control the bleeding. The patient has to be immediately resuscitated with fluids and blood products must be given as needed. It is advisable to use a combined management approach with intraoperative endoscopy to manage gastrointestinal bleeding with care to avoid disrupting a newly created anastomosis. Surgical management consists of either laparoscopy or laparotomy. If the patient is profoundly hypotensive, laparoscopy is relatively contraindicated.
5.5 Prevention
Confirm Blood Type (based on two or more independently collected samples): Order a “Type and Screen” for every patient who is undergoing bariatric surgery. The specimen may be drawn up to 30 days in advance of surgery.
Thorough examination through the patient’s history and on his preoperative laboratory results in the preoperative assessment. Investigate the patient’s history and his family’s history of bleeding. If any suspicion of higher risk for bleeding, he should be referred to a hematology specialist and receive a more thorough investigation. Afterwards, the patient should return as an outpatient, with recommendations of preventions for bleeding, if needed.
Mechanical and chemical prevention, making sure to obtain hemostasis in all staple-line edges.
Consider reinforcing the staple-line in sleeve gastrectomy using bovine pericardium, synthetic polyester or glycolide/trimethylene or oversewing. This may enhance homeostasis and reduce bleeding incidence when compared to no reinforcement [47–49].
Using the correct staple height for a given tissue is one of the most important factors to limit this complication [50]. Use thicker staple sizes for the stomach and thinner staple sizes for the small bowel [18].
References
1.
2.
Nguyen NT, Rivers R, Wolfe BM. Early gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg. 2003;13(1):62–5.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree