A second type of dialysis-related hypotension is a chronic, persistent form of hypotension that occurs in approximately 5% of dialysis patients (1). This second form of hypotension is usually observed in patients who have been on hemodialysis (HD) for at least 5 years. Such patients often come to the dialysis unit with a systolic blood pressure of less than 90 mm Hg. This form of hypotension is associated with an increased mortality and is often a manifestation of malnutrition and/or cardiovascular disease in the chronic HD patient (3–5). In one recent review of a group of such patients, chronically hypotensive patients had a lower peripheral resistance than normotensive patients (6). Of interest is that in this study, cardiac output was not significantly elevated in the hypotensive group, perhaps suggesting some decrease in cardiac reserve. This chapter focuses on the problem of episodic hypotension, which is the most frequent form of HD-related hemodynamic instability.
 OVERVIEW OF THE PROBLEM
OVERVIEW OF THE PROBLEM
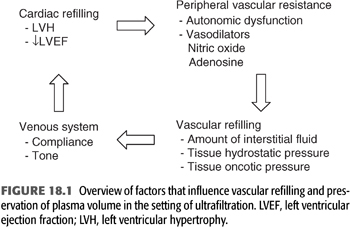
Dialysis hypotension is the result of an inadequate cardiovascular response to the reduction in blood volume that occurs when a large volume of water is removed over a short period of time. In a typical dialysis procedure, an ultrafiltrate volume that is equal to or greater that the entire plasma volume is often removed. Despite the large ultrafiltrate volume, plasma volume typically decreases by only approximately 10% to 20% (7). This ability to maintain plasma volume during ultrafiltration requires mobilization of fluid from the interstitial into the intravascular space. Adequate tone and normal compliance characteristics of the venous system is required to redistribute this fluid into the central circulation where it becomes available for cardiac refilling. Once delivered to the central circulation, normal systolic and diastolic function of the heart ensures maintenance of cardiac output. Should cardiac output begin to decline, blood pressure is stabilized by sympathetic nervous system–induced increases in peripheral vascular resistance.
Disturbances in either one or several of these steps will render the patient prone to develop hypotension in the setting of fluid removal (FIGURE 18.1). These disturbances can be the result of the chronic uremic state as well as accompanying comorbid conditions. The following sections review the various abnormalities in the autonomic and cardiovascular systems that contribute to the development of episodic dialysis hypotension. The chapter concludes with a discussion of the prevention and management of dialysis-related hypotension.
 VASCULAR REFILLING
VASCULAR REFILLING
The ability to maintain plasma volume during ultrafiltration requires mobilization of fluid from the extravascular into intravascular space. The success of vascular refilling is influenced by the stability of plasma osmolality, the rate of fluid removal, and other patient characteristics that dictate the distribution of fluid between the body fluid compartments.
Stable Plasma Osmolality
The importance of a stable plasma osmolality as a key element in preventing dialysis hypotension is discussed in detail concerning dialysate composition. The main mechanism by which a stable plasma osmolality contributes to hemodynamic stability appears to be through the maintenance of a more stable extracellular fluid volume and plasma volume (FIGURE 18.2) (8). The rapid fall in plasma osmolality that results from solute removal leads to the movement of water to intracellular loci. This movement of water will decrease the amount of fluid accessible for vascular refilling. The use of a higher dialysate sodium concentration (greater than 140 mEq/L) is an effective means to assure adequate vascular refilling and has proved to be among the most effective and best-tolerated therapies for episodic hypotension. A high-dialysate sodium concentration limits the decline in plasma osmolality and therefore leads to the removal of volume during ultrafiltration from both intracellular and extracellular compartments. This effect limits the compromise in the plasma volume that normally occurs during rapid falls in plasma osmolality coupled with a very large ultrafiltration rate. Another potential mechanism by which a decline in plasma osmolality may contribute to dialysis hypotensive episodes is by impairing peripheral vasoconstriction during volume removal (9,10). A decline in plasma osmolality may also adversely affect baroreceptor function by blunting afferent sensing mechanisms, thereby contributing to autonomic dysfunction (9).

Local Starling Forces
Several patient characteristics that affect Starling forces operating at the capillary level influence the process of vascular refilling. One such characteristic is the amount of interstitial fluid present, a parameter clinically reflected by the dry weight of the patient. When the volume of interstitial fluid is small, any ultrafiltrate volume will more likely be associated with hemodynamic instability. This explains the development of hypotension when patients are dialyzed below their true dry weight. By contrast, increased amounts of interstitial fluid will expand the volume of fluid accessible for refilling of the intravascular space and, therefore, decrease the likelihood of hypotension. In most patients, a dry weight is selected that minimizes the amount of interstitial fluid present because chronic volume overload has long-term deleterious effects on the cardiovascular system. However, in patients with recurrent IDH or chronic persistent hypotension that is not amenable to other interventions, it may be necessary to purposely maintain the patient in a hypervolemic state so that the dialysis procedure can be employed with a lower likelihood of hemodynamic instability.
Determinants of the oncotic and hydrostatic pressure at the tissue level will also affect vascular refilling. For example, a well-nourished patient with a normal serum albumin concentration is more likely to have better preserved vascular refilling as compared to a patient with hypoalbuminemia. On the other hand, administration of a vasodilator has the potential to impair vascular refilling by allowing excessive transmission of arterial pressure into the capillary bed resulting in increased hydrostatic pressure.
 CARDIAC REFILLING AND VENOUS DYSFUNCTION
CARDIAC REFILLING AND VENOUS DYSFUNCTION
When vascular refilling and plasma volume are normal, IDH can still occur if there is a failure to properly redistribute intravascular volume into the central circulation (11). In some patients, this failure can be traced to functional and structural abnormalities on the venous side of the circulation.
Because most plasma volume is located on the venous side of the circulation, even a small decrease in tone of the venous system can potentially limit cardiac filling because of a decrease in central blood volume. Abrupt splanchnic vasodilation can be the cause of IDH. Such vasodilation may result from ingestion of food during the dialysis procedure or withdrawal of sympathetic tone resulting from an increase in core body temperature. Increased production of vasodilators such as adenosine or nitric oxide may also play a role in this complication.
Decreased venous compliance has also been demonstrated in hypertensive HD patients (12,13). Such a disturbance in venous compliance can lead to hemodynamic instability in at least two ways. First, decreased venous compliance leads to a steep volume-to-pressure relationship such that a major drop in cardiac filling pressure can occur with only a small decrease in plasma volume. In this regard, an inverse relationship has been observed between venous compliance and the fall in central venous pressure in dialysis patients during isolated ultrafiltration (12). Second, impaired venous compliance can lead to higher hydrostatic pressures in the upstream capillary bed resulting in reduced vascular refilling from the interstitium. Patients with evidence of decreased venous compliance exhibit a greater decrease in plasma volume during ultrafiltration as compared to those subjects with normal venous compliance characteristics (12,13). The basis for altered venous function in chronic uremia may relate to structural abnormalities of the venous wall. Morphologic studies of the iliac and inferior caval veins of hypertensive patients with end-stage kidney disease (ESKD) have shown increased thickness of the media in the venous wall (14). Such structural abnormalities may also impair the ability to mobilize erythrocytes from the splanchnic or splenic circulation into the systemic circulation during ultrafiltration (15). This mechanism may be particularly important in patients who also exhibit impaired autonomic function.
 SYSTOLIC AND DIASTOLIC FUNCTION OF THE HEART
SYSTOLIC AND DIASTOLIC FUNCTION OF THE HEART
As the central circulation is filled, hemodynamic stability then becomes dependent on normal systolic and diastolic function of the heart to generate an adequate cardiac output in the face of volume removal. Myocardial dysfunction is commonly present in patients with ESKD and can be an important cofactor in dialysis-induced hypotension (16).
Diastolic Dysfunction
The frequent occurrence of hypertension and on occasion aortic stenosis contributes to pressure overload of the left ventricle and accounts for the frequent occurrence of concentric left ventricular hypertrophy (LVH). Other contributing factors include the presence of an arteriovenous fistula, anemia, and intermittent bouts of volume overload.
Left ventricular (LV) dilatation and hypertrophy lead to changes in ventricular performance that differ strikingly from that seen in the healthy heart. A high incidence of LVH and abnormal diastolic function leads to diminished diastolic distensibility, such that the volume–pressure relationship during diastolic filling is steeper and shifted to the left (FIGURE 18.3). When LV compliance is diminished, a small rise in left ventricular end-diastolic volume (LVEDV) may cause a disproportionately large increase in left ventricular end-diastolic pressure (LVEDP) with consequent pulmonary venous congestion but little or no increase in stroke volume. Even in the presence of well-preserved systolic function, the preload reserve of the left ventricle can be exceeded by abrupt or large increases in plasma volume or because decreased ventricular compliance leads to inappropriately high filling pressure and pulmonary congestion. Furthermore, decreases in plasma volume, as during ultrafiltration in the setting of a steep volume–pressure relationship, can critically decrease the filling pressure of the heart and result in hypotension. In short, many patients with ESKD operate within a narrow LV volume–pressure relationship such that changes in plasma volume are often not well tolerated.
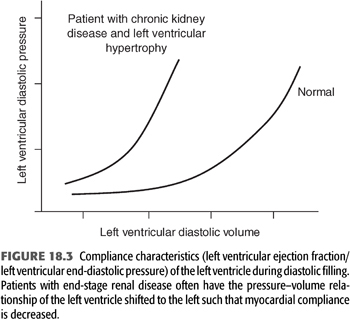
The contribution of LVH to hemodynamic instability during dialysis is highlighted by a number of studies that have shown LVH to be more common in dialysis patients who experience episodic dialysis hypotension (17). Ritz et al. (18) observed that the LV mass-to-volume ratio was 37% greater in 27 patients with recurrent IDH than in 27 patients without this complication. Similarly, Wizemann et al. (19) noted that the incidence of dialysis hypotension was several times greater in patients with echocardiographic LVH than in those with normal ventricular mass. Another study found patients with recurrent IDH had a lower predialysis blood pressure, more severe concentric LVH, and lower LV compliance (20).
Patients with diastolic dysfunction are particularly difficult to treat. In the setting of impaired ventricular relaxation, a small amount of volume replacement can potentially trigger pulmonary edema. Therapy with inotropic agents can exacerbate the problem by further impairing ventricular relaxation. Calcium channel blockers such as verapamil or diltiazem may have some utility in this setting because these agents exert a negative inotropic effect on the heart and have the potential to improve LV relaxation. In a small series of patients, use of these drugs produced a functional improvement in cardiac performance and a reduction in the frequency of hypotension (21). However, it must be emphasized that the use of vasodilator calcium channel blockers has the potential disadvantage of lowering blood pressure. Hence, the trade-off of improving LV relaxation may not be tolerated in all patients because of the effect of the drugs to lower blood pressure.
On a more chronic basis, therapy can be instituted with the goal of reducing LV mass toward normal and presumably improve ventricular diastolic function. Partial or complete regression can be achieved by control of hypertension and by correction of anemia with erythropoietin (22). Studies in nonuremic hypertensive subjects suggest that, at equivalent blood pressure control, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers reduce LV mass more rapidly and perhaps more effectively than most other antihypertensive drugs. It is not clear if these observations apply to the patient with renal failure. Correction of anemia with erythropoietin presumably acts by improving tissue oxygen delivery, thereby allowing the cardiac output (which is increased in anemia) and, therefore, cardiac work to fall toward normal. These changes have been associated with a 10% to 30% reduction in LV mass index (22). However, attention must also be paid to the frequent elevation in blood pressure following erythropoietin, an effect that may partially counteract the benefit associated with the elevation in hematocrit. In addition, one needs to be cautious not to increase the hemoglobin concentration to values in excess of 12 g/L because higher values have been associated with an increase in cardiovascular events (23,24).
Systolic Dysfunction
Impaired systolic function of the heart is common in dialysis patients. Depressed systolic function may be the result of a prior myocardial infarction, ischemic or hypertensive cardiomyopathy, or ischemic injury resulting from microvascular disease as in diabetes mellitus. Some patients with this disorder manifest the chronic persistent form of hypotension. More commonly impaired systolic function contributes to an increased frequency of dialysis-associated hemodynamic instability. Decreased myocardial contractility leads to poor LV performance and, importantly, a diminished cardiac reserve in the context of a hemodynamic challenge. Patients prone to hypotension during dialysis exhibit a blunted increase in cardiac index in response to a dobutamine infusion as compared to those subjects without hemodynamic instability (25). An inability to generate a sufficient cardiac output despite adequate cardiac filling can contribute to IDH and pulmonary edema.
 ARTERIAL DYSFUNCTION
ARTERIAL DYSFUNCTION
An increase in vascular resistance on the arterial side of the circulation is an important defense against the development of hypotension when cardiac output begins to fall. Disturbances in this response can contribute to hemodynamic instability through several mechanisms. Impaired autonomic function or increases in core body temperature can lead to decreased arterial tone and result in more direct transmission of systemic pressure into the venous circulation. A sudden increase in venous pressure will increase the holding capacity of the venous compartment potentially leading to the sequestration of fluid thereby limiting cardiac refilling (7).
Disturbances in arterial function can also be the result of structural abnormalities in the arterial circulation. Vascular lesions in arteries of patients with uremia are characterized by intimal fibrosis and medial calcifications with little to no deposition of lipid droplets (26). These changes tend to be more reminiscent of accelerated aging or diabetic macroangiopathy rather than typical atherosclerosis. The causes of these pathologic changes are presumably related to age, effects of hypertension, and abnormalities of calcium and phosphorus metabolism. Such changes lead to increased wall stiffness and importantly contribute to the development of LVH.
Adenosine
Arteriolar vasoconstriction mediated by increased sympathetic nerve activity maintains blood pressure by increasing peripheral vascular resistance. In some patients, this response is inadequate due to production of vasodilators. In the setting of hypotension, no matter what the cause, tissue ischemia will result in net negative balance between the synthesis and degradation of adenosine triphosphate (ATP). As a consequence, ATP metabolites will begin to accumulate and will be released into the extracellular fluid. One such metabolite of ATP is adenosine (FIGURE 18.4) (27). Adenosine is an endogenous vasodilator released by endothelial cells and vascular myocytes and has been implicated in the sudden onset of decreased blood pressure during dialysis. In an attempt to demonstrate that accumulation of adenosine during dialysis-induced hypotension is more than just a marker of the ischemic event, Shinzato et al. (27) measured the metabolites of ATP before, during, and after dialysis-induced hypotension in a group of hypotensive-prone chronic dialysis patients. In this study, plasma levels of inosine, hypoxanthine, and xanthine rose sharply with the development of sudden IDH and quickly decreased as the blood pressure was restored. By contrast, there was no significant variation in the plasma levels of the metabolites when hypotension developed more gradually during the course of the procedure. Adenosine levels were not measured because of the short half-life of the metabolite. These patients were then treated with caffeine, a nonselective A1 and A2 adenosine-receptor blocker, and the frequency of hypotensive episodes was examined. A 250-mg capsule of caffeine administered 2 hours into the procedure resulted in a significant decrease in the development of hypotensive episodes.
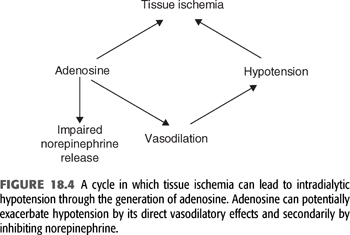
In a prospective double-blind placebo-controlled trial in patients with frequent IDH, injection of a selective A1 receptor blocker provided a significant but modest benefit in reducing the incidence of hypotension (28). Expression of the A2A receptor is increased to a greater extent on peripheral blood mononuclear cells in dialysis patients with frequent hypotension compared to hemodynamically stable patients; suggesting A2A receptors antagonism might also be of benefit (29).
Nitric Oxide
Another intriguing and as yet ill-defined contributor to the development of HD hypotension is that of nitric oxide (30). It has been proposed that blood bioincompatibility with dialysis membranes leads to the activation of monocytes in the peripheral circulation. This is thought to produce a variety of cytokines including interleukin 1 (IL-1) and tumor necrosis factor (TNF). These cytokines in turn induce the synthesis of nitric oxide by endothelial cells. The support for this hypothesis is derived from several sources. First, plasma levels of IL-1 and TNF are elevated in patients with ESKD on dialysis (31). Furthermore, IL-1 and TNF induce hypotension when administered in vivo, predominantly as a result of a decline in systemic vascular resistance (32). In vitro studies have also shown that IL-1 and TNF promote nitric oxide synthesis in endothelial cells. Nitric oxide has a direct effect as a smooth muscle vasodilator, working through intracellular cyclic guanylyl monophosphate (GMP) as a second messenger. In addition, IL-1 is also capable of inducing the synthesis of vasodilator prostaglandins (PGs), including PGE2 and PGI2 in human vascular smooth muscle cells and endothelial cells (33). These arachidonic acid metabolites are direct smooth muscle vasodilators as well and could thereby contribute to a decline in peripheral vascular resistance.
The concentration of the stable end products of endogenously released nitric oxide, nitrite and nitrate, is increased in chronic kidney disease patients undergoing HD as compared with healthy controls (34,35). Normally, these levels decrease significantly during a dialysis treatment in otherwise hemodynamically stable patients. By contrast, studies show these metabolites actually increase in patients who develop IDH (34,35). A higher predialysis fractional exhaled nitric oxide concentration has also been found in patients with IDH as compared with those with stable hemodynamics (35). These findings suggest that increased synthesis of nitric oxide may be contributing to hemodynamic instability in hypotensive-prone subjects. The basis for increased nitric oxide activity is unknown but may be related to the dialytic removal of the nitric oxide inhibitor, asymmetric dimethylarginine (ADMA) (36). If further studies confirm an important role for increased synthesis of nitric oxide in dialysis-associated hemodynamic instability, administration of an antagonist to nitric oxide production such as N(G)-monomethyl-arginine acetate (l-NMMA) may ultimately prove useful in the treatment of hypotensive-prone patients.
 AUTONOMIC NEUROPATHY
AUTONOMIC NEUROPATHY
Autonomic Dysfunction in Uremia
The importance of autonomic dysfunction as a contributing factor to dialysis hypotension has been the subject of vigorous investigation for the last 15 years. One of the first clinical associations between impaired autonomic nervous system control and hypotension was described by Bradbury and Eggelston (37) in 1925. They observed that the autonomic nervous system was essential in the orthostatic regulation of arterial pressure through baroreflex mechanisms. Subsequently, the central and peripheral neuronal pathways that control vasomotor tone, heart rate, myocardial contractility, and venous capacitance have been described in greater detail.
An autonomic reflex arc is composed of an afferent limb that consists of sensory nerve fibers originating from the vascular tree, visceral organs, and the skin. These afferent fibers travel to the central nervous system mainly through the spinal cord and vagus nerves. Arterial baroreceptors are located in the aortic arch and carotid sinus and sense changes in blood pressure; cardiopulmonary baroreceptors are located in the atrium, ventricles, great veins, and pulmonary vessels and sense changes in cardiac filling pressures. Information about these pressure changes is integrated in the central nervous system at several different levels including the brainstem, cerebellum, hypothalamus, and cerebral hemispheres. The efferent limb of the system consists of both sympathetic and parasympathetic pathways that exit the brainstem to the spinal cord and travel to the heart and circulation.
Abnormalities in autonomic function are commonly present in patients with chronic kidney disease (38,39). The presence of these abnormalities can potentially impair the patient’s ability to maintain systemic blood pressure following a large degree of fluid removal by ultrafiltration. One such abnormality is an inadequate sympathetic response to volume removal in patients with recurrent IDH. The postdialysis plasma concentration of chromogranin A (a protein coreleased with catecholamines) increases to a lesser extent in patients with IDH compared to those with stable blood pressure consistent with a blunted response of sympathetic nerve activity to hypotension (40).
Under normal conditions, sympathetic neural outflow is under tonic restraint by baroreceptors in the aortic arch and carotid sinus and by baroreceptors in the cardiopulmonary region. With the volume removal that accompanies HD, reductions in central venous pressure and arterial blood pressure would be expected to unload these baroreceptors and thereby reduce their tonic restraint on the vasomotor center, resulting in reflex increases in sympathetic outflow to the heart and peripheral circulation to help maintain blood pressure. Defects in this homeostatic response may play an important role in the development of hypotension during the dialytic procedure.
Numerous tests have been employed to assess the autonomic nervous system in patients with chronic kidney disease. These tests may be used to try to localize the defect in the autonomic nervous system. For example, the Valsalva test probes the integrity of the entire autonomic nervous system: low-pressure and high-pressure baroreceptors in the cardiopulmonary circulation, the afferent and efferent limbs of these pathways, and both sympathetic and parasympathetic function. This test is, therefore, useful in detecting a defect but not in identifying the site of the abnormality. The amyl nitrite inhalation test may be used to test low-pressure baroreceptors and the resultant efferent sympathetic outflow expected when blood pressure declines. The cold pressor test (performed by placing a cold cloth on the patient’s forehead or by submerging a hand in ice slush) predominantly reflects efferent sympathetic function activated by cold-induced peripheral vasoconstriction. The precise role of the autonomic nervous system in dialysis patients has been difficult to assess in part because tests of autonomic function have not been routinely included in large surveys of dialysis subjects. For this reason, the natural history of changes in the autonomic nervous system in subjects with ESKD is not presently clear.
The initial study that suggested that a defect in the autonomic nervous system could be related to HD hypotension was published in 1976 by Lilley et al. (41). These investigators performed a variety of autonomic tests and concluded that the defect in the autonomic nervous system in patients with ESKD was on the afferent side of the autonomic loop. More specifically, they concluded that the defect resided in the baroreceptors. The finding of autonomic dysfunction at the levels of the afferent branch of the baroreflex arc is consistent with numerous other studies published in the literature in which selective testing of the vasomotor center and the efferent sympathetic tract (cold pressor test, handgrip test, mental stress) showed normal function (41,42), whereas tests of the complete baroreflex arc showed a diminished response (41–44).
The precise mechanism of the autonomic dysfunction remains unclear but, because efferent sympathetic function appears to be normal, the most likely sites of primary damage are the baroreceptors or their afferent fibers or central nervous system connections. Chronic fluid overload leading to persistent overstretching has been suggested as one cause of baroreceptor dysfunction (45). In addition, there has been some sentiment that uremic “poisoning” of baroreceptors is involved in dialysis hypotension (46,47). Chronic exposure to uremic toxins has been thought to poison the baroreceptors, leading to almost complete removal of their tonic inhibitory effects on sympathetic vasomotor outflow in some patients (41,46). According to this hypothesis, uremia-induced sinoaortic baroreceptor deafferentation would cause central sympathetic outflow to be nearly maximal under basal conditions so that it could not possibly increase much further during the hypotensive stress of HD. However, the experimental support for this hypothesis is indirect, relying mainly on plasma catecholamines and semiquantitative bedside tests to assess sympathetic neural function, and a definitive relation between impaired baroreflexes and dialysis-induced hypotension has not been established. Chronic hyperkalemic depolarization of nerves has also been suggested as playing a contributory role (48).
Paradoxical Withdrawal of Sympathetic Tone in Dialysis-Induced Hypotension
To better characterize the role of autonomic dysfunction in the genesis of IDH, Converse et al. (49) examined baroreflex control of heart rate, efferent sympathetic nerve activity, and vascular resistance in 16 hypotension-resistant and 7 hypotension-prone HD patients. Hypotension-prone patients were defined as those in whom a sudden, symptomatic decrease in mean arterial pressure of greater than 30 mm Hg occurred during at least one-third of maintenance HD sessions. Patients with diabetes were excluded from the study because autonomic neuropathy is a well-known complication of diabetes mellitus. Direct measurements of sympathetic nerve activity and several quantifiable reflex maneuvers were employed to test the effects of chronic uremia on a number of specific reflexes, including arterial baroreflex control of heart rate and cardiopulmonary baroreflex control of vascular resistance. By studying the same patients both during the interdialytic period and during actual sessions of maintenance HD, these investigators were able to separate the autonomic effects of chronic uremia from the acute autonomic effects of the HD procedure.
To assess whether a chronic abnormality was present in either arterial or cardiopulmonary baroreceptors, both groups of patients were first studied during the interdialytic period. Measurement of heart rate and sympathetic nerve activity during the intravenous infusion of nitroprusside and measurement of forearm vascular resistance during the application of negative pressure to the lower body disclosed no abnormalities in either group of patients. These results suggested that there was no baseline autonomic defect chronically present to account for dialysis-induced hypotension in this select group of nondiabetic patients. It should be pointed out that in other groups of dialysis patients, such as those with diabetic autonomic neuropathy, impaired baroreflexes indeed may contribute more to hypotension during HD.
Studies were then undertaken to examine autonomic function during the dialytic procedure. In a subgroup of patients who developed severe hypotension on dialysis, baroreceptor function was again normal, that is, a small initial decrease in blood pressure was accompanied by the appropriate reflex increases in sympathetic nerve activity, heart rate, and peripheral vascular resistance. As the hypotensive episode became severe, however, normal baroreflex function was replaced by the sudden appearance of an inappropriate vasodepressor reaction. With the additional fall in blood pressure, sympathetic activity, heart rate, and vascular resistance did not increase further but rather fell paradoxically back to or below baseline levels. Shortly after the onset of the hypotension and loss of sympathetic activation, classic signs and symptoms of vasovagal syncope developed in the patients, including nausea, abdominal discomfort, diaphoresis, and giddiness.
To further examine the mechanism triggering this vasodepressor reaction, additional experiments were performed in which the normal HD procedure was separated into its component parts, ultrafiltration alone and dialysis alone. Using measurements of calf vascular resistance, it was found that ultrafiltration reproduced both the increases and decreases in vascular resistance (including vasodilation and hypotension), whereas dialysis alone had no effect on calf vascular resistance in either group of patients. These results suggested that withdrawal of volume appears to be the key stimulus triggering this vasodepressor reaction, which in turn exacerbates the volume-dependent fall in blood pressure.
An inhibitory reflex arising in the heart is the most likely mechanism causing this paradoxical bradycardia and vasodilation during hypovolemic hypotension (50–52). In addition to being a pump, the heart is also a sensory organ, being richly innervated with sensory (or afferent) nerves. Many of these sensory nerves function as mechanoreceptors and therefore signal the brain of changes in loading conditions and contractility of the ventricles. Their function is to inhibit sympathetic outflow. During euvolemia, these sensory nerves normally exert a tonic inhibitory influence on sympathetic vasomotor outflow. During mild hypovolemia, this inhibitory influence is reduced. During severe hypovolemia, however, this inhibitory influence is not reduced further but instead increases paradoxically. The theory is that the receptive fields of these endings are deformed as the adrenergically stimulated heart contracts forcefully around an almost empty ventricular chamber.
It was hypothesized that mild hypovolemia might simulate nonhypotensive HD: unloading of inhibitory ventricular afferents causing reflex sympathetic activation. In contrast, severe hypovolemia might simulate HD-induced hypotension: paradoxical activation of inhibitory ventricular afferents causing reflex inhibition of sympathetic outflow resulting in bradycardia and peripheral vasodilation. Echocardiographic measurements of LV volumes before the onset of severe hypotension showed near-obliteration of the LV cavity possibly accounting for the paradoxical activation of inhibitory LV afferents.
Therefore, in a subset of hypotensive-prone dialysis patients, a form of acute autonomic neuropathy plays an etiologic role. For this paradoxical sympathetic failure to occur, severe volume depletion must occur. To determine the prevalence of this type of hypotension (bradycardic hypotension) in the dialysis population, Zoccali et al. (53) identified 20 patients out of a total population of 106 who suffered from IDH. In 60 hypotensive episodes recorded in the 20 patients, heart rate increased in 35 episodes, remained unchanged in 19 episodes, and decreased in 6 episodes. The five patients who developed bradycardic hypotension were characterized by high ultrafiltration rates and smaller LV end-diastolic diameters as compared with those with tachycardic or fixed heart rate.
Autonomic Dysfunction and Chronic Hypotension
As mentioned previously, a small fraction of HD patients have difficulty maintaining a normal blood pressure and are chronically hypotensive between dialysis treatments. The role of autonomic dysfunction in this condition was examined in one study that compared numerous measures of autonomic function among hypotensive HD patients, normotensive HD patients, and control patients (all groups consisted of 17 individuals) (54). Chronically hypotensive individuals exhibited a significant downregulation of α- and β-adrenergic receptors, suggesting an inability to produce an adequate sympathetic response.
Autonomic Dysfunction and Arrhythmias
The development of cardiac arrhythmias in ESKD can be traced to several factors that are operative alone or in combination and include the presence of myocardial dysfunction, fluid and electrolyte shifts, and poor oxygen saturation. A previously unrecognized predisposing factor may be autonomic dysfunction. One study of 41 dialysis patients evaluated the correlation between arrhythmia (as determined by 24-hour Holter examination) and autonomic dysfunction (as determined by blood pressure and heart rate responses) (55). Compared with patients with normal autonomic function, a significantly increased incidence of atrial and/or ventricular arrhythmia was found among those with one or more autonomic abnormalities (41 abnormal rhythms in 26 patients with autonomic dysfunction vs. 1 in 15 with normal autonomic responses).
Sympathetic Nervous System and Hypertension in the Uremic State
Despite the frequent presence of autonomic dysfunction, baseline plasma catecholamine levels are often elevated in chronic kidney disease (56). Increased sympathetic tone, decreased degradation, and diminished neuronal reuptake all may contribute to this finding. The physiologic significance of this finding is uncertain, but sympathetic overactivity could contribute to the common development of hypertension in ESKD. The signal for this increased sympathetic tone may in part originate with stimulation of renal afferent nerves.
Chemosensitive renal afferent nerves have been implicated in the pathogenesis of hypertension by causing reflex activation of sympathetic outflow to the heart and peripheral circulation (57–59). Stimulation of these afferent nerves by either ischemic metabolites, such as adenosine, or by uremic toxins, such as urea, evokes reflex increases in sympathetic nerve activity and blood pressure in experimental animals (60). Reduced sympathetic activity may be one important mechanism by which bilateral nephrectomy lowers blood pressure in some HD patients (61).
In recent years, additional tests of autonomic dysfunction have been assessed. Principle among these is heart rate variability, a noninvasive measure of the autonomic system (62,63). In the most extensive of these studies, a lower heart rate variability was associated with progression to ESKD and poorer outcome. Of interest is the fact that at least one study (64) demonstrated improvement in heart rate variability after successful renal transplantation, suggesting that, to some degree, autonomic dysfunction in ESKD patients is reversible.
 THERAPY FOR HEMODYNAMIC INSTABILITY DURING HEMODIALYSIS
THERAPY FOR HEMODYNAMIC INSTABILITY DURING HEMODIALYSIS
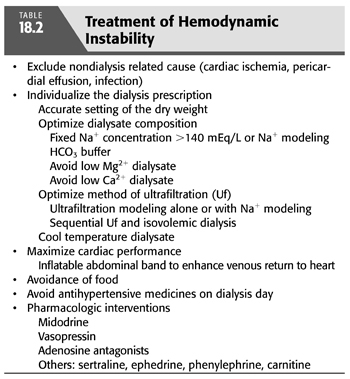
In patients in whom dialysis hypotension has not been a problem but in whom it develops suddenly, the differential diagnoses must be expanded to include occult septicemia and unrecognized cardiac or pericardial disease. In this regard, the exclusion of a pericardial effusion and tamponade and/or a significant segmental wall motion abnormality are important in the assessment in any patient with hypotensive episodes occurring frequently. Other serious underlying conditions that can give rise to new-onset hypotension include intestinal ischemia with impending bowel infarction and occult hemorrhage. Hypotension during the early part of the procedure should make one consider exaggerated cytokine release resulting from a reaction with the dialysis membrane. After excluding these processes, there are several options available for the treatment and prevention of episodic dialysis hypotension (TABLE 18.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


