Fig. 13.1
Collapsing glomerulopathy seen in HIVAN. Jones-methenamine stain showing collapse of the capillary loops with overlying podocyte hyperplasia (×40)
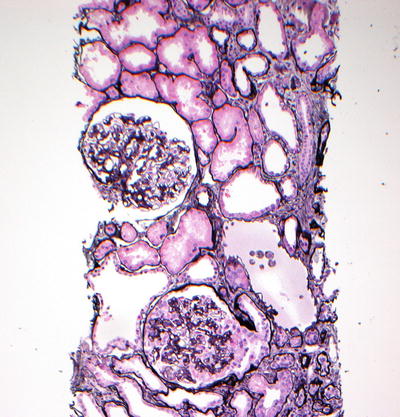
Fig. 13.2
Low-power view of collapsing glomerulopathy in HIVAN. Jones-methenamine stain showing collapse of the capillary loops with overlying podocyte hyperplasia in the lower glomerulus (×20)
Types of Kidney Disease in HIV
The underlying etiologies of CKD in persons with HIV can be categorized as follows: (1) HIV-associated nephropathy (HIVAN), (2) HIV-related glomerular diseases that are not HIVAN including immunodeficiency renal diseases, (3) drug-induced nephrotoxicity, and (4) CKD related to non-HIV comorbidities such as diabetes mellitus or hypertension [33]. The first account of kidney disease associated with HIV was in 1984 as a description of what is known today as focal segmental glomerulonephritis [34, 35]. Regardless of etiology, persons with kidney disease have a greater risk of death and cardiovascular events when compared with the community-based population without CKD, a phenomena that still applies in the HIV-infected community with CKD [21, 36].
HIV-Associated Nephropathy (HIVAN)
HIVAN was first described on renal biopsy as collapsing focal glomerulosclerosis [34, 35]. However, in the largest biopsy series of HIV-infected patients of African descent, it has more recently been defined as a histological spectrum, a “constellation of glomerular, interstitial, and tubular abnormalities,” albeit with a glomerular predominance [37]. Table 13.1 describes the changes that can be seen in the nephron which correspond to a histological diagnosis of HIVAN, including tubular microcysts, mesangial hyperplasia, interstitial fibrosis, and lymphocytic infiltrates [6, 37]. HIVAN has been traditionally reported as a late manifestation of HIV but has occasionally been described earlier in the disease [6]. Clinically, it is characterized by variable proteinuria, renal failure, large echogenic kidneys, and absence of peripheral edema and can rapidly progress to ESRD [6, 7]. The progression of HIVAN to ESRD can be significantly slowed but not completely prevented by HAART therapy [6]. In addition to ethnicity, risk factors for the progression of HIVAN likely include lower CD4+ T cell count (<200 cells/μL), higher HIV RNA level, and severe renal failure on presentation [5, 38]. There are also genetic associations that have been explored earlier in this chapter. In the study by Wearne et al. [37], without antiretroviral therapy, the expected median survival after biopsy confirmed HIVAN was approximately 4.5 months, with the “fetal variant” having the worst prognosis [37].
Table 13.1
Histological changes in the nephron seen with HIVAN
Glomerular changes | Interstitial changes | Tubular changes |
|---|---|---|
FSGS, collapsing variant | Fibrosis | Presence of microcysts |
Global sclerosis with epithelial cell involvement (fetal variant) | Lymphocytic infiltrate | Epithelial cell hyperplasia and hypertrophy |
FSGS, non-collapsing varianta | Plasma cells within the lymphocytic infiltrate | |
Diffuse inflammatory lymphocytic syndrome |
HIV-Related Glomerular Diseases That Are Not HIVAN
While biopsy studies suggest that HIVAN represents approximately one-third of the glomerular disease among African Americans with lower CD4+ T cell counts, it should be emphasized that it is uncommon in Caucasians and Asians; thus there must be other HIV-related glomerular disease seen more often in these ethnic groups and those with higher CD4+ T cell counts [11, 17, 21]. These include HIV-immune complex disease like membranoproliferative glomerulonephritis (MPGN; including hepatitis C associated), lupus-like glomerulonephritis, IgA nephropathy, membranous nephropathy (MN), and HIV-thrombotic microangiopathies [6, 11, 17]. The HIV-immune complex renal disease involves the deposition of circulating immune complexes containing HIV antigens with B cell lymphocytic infiltrates (compared to T cell infiltrates in HIVAN), inflammation, and scarring [6]. It has been reported in up to 80 % of Caucasians with HIV and glomerular disease [5]. The benefit of HAART therapy on slowing the progression of the HIV-immune complex kidney disease is less clear compared to that seen in HIVAN. While some studies of IgA nephropathy demonstrate that HIV antigens may play an important and causative role [39] in the pathogenesis of these lesions, the definitive evidence linking HIV to these lesions is not available. The HIV-related lupus-like glomerulonephritis involves the subendothelial deposition of multiple immune complexes including IgA, IgG, IgM, C3, and C1q in the absence of serologic and clinical diagnosis of systemic lupus erythematosus (SLE) [40]. HIV-thrombotic microangiopathy (hemolytic uremic syndrome or thrombotic thrombocytopenic purpura) is rare but, when identified, predominates among Caucasians; like its non-HIV-associated counterparts, histological features include the deposition of microthrombi in the glomerular capillaries, fibrinoid necrosis, and onion skinning [6].
Anti-neutrophil cytoplasmic antibodies (ANCA) have been noted in the sera of HIV-infected individuals with varying frequency (18–41.0 %) in the absence of clinical features suggestive of a vasculitis [41–43]. A review of the literature finds only two case reports of persons with HIV who developed an active ANCA-positive vasculitis [42, 44, 45]. Similarly, only one case report of anti-GBM disease in a patient with HIV has been published [46]. The finding of these two autoantibodies in the absence of kidney disease suggests that their pathophysiologic effects may be modified in the presence of HIV infection. Positive tests for these antibodies should be interpreted with caution and should not be used to decide on empiric therapy in the absence of a kidney biopsy to confirm histology.
Drug-Induced Nephrotoxicity
While the treatment of HIV with antiretroviral medications has therapeutic benefit in HIVAN, the medications can also result in acute kidney injury (AKI) due to acute tubular necrosis, allergic interstitial nephritis, and obstructive uropathy [21, 36]. Table 13.2 lists common HIV medications and their potential renal toxic effects. Tenofovir, indinavir, and atazanavir are the most frequently described as having nephrotoxic effects. Tenofovir is a nucleoside reverse transcriptase inhibitor, and while early clinical trials did not reveal appreciable nephrotoxicity, there have been numerous post-marketing reports describing renal tubular injury with this agent [47]. Tenofovir is actively secreted into the tubules and damages the proximal tubules by a direct cytotoxic effect; this eventually causes a Fanconi syndrome and potential renal tubular acidosis [48]. These phenomena have been seen more frequently in cases where tenofovir was administered as part of a regimen containing HIV protease inhibitors such as ritonavir or lopinavir as these increase the maximum serum concentration of tenofovir by >30 % [6, 49]. Tenofovir nephrotoxicity is largely reversible with the removal of the offending drug. The HIV protease inhibitor indinavir causes nephrotoxicity by inducing crystal formation in approximately 20 % of patients receiving it; this subsequently leads to obstructive uropathy and drug-induced interstitial nephritis [6, 50]. Indinavir also reduces the production of nitric oxide which is a potent vasodilator. A reduced amount of this molecule in the kidney vessels therefore leads to vasoconstriction and subsequent ischemia, which is another proposed mechanism of indinavir-mediated renal injury [51]. Patients can be asymptomatic or present with classic symptoms of dysuria and flank pain and the urinalysis will reveal crystalluria and pyuria; renal ultrasound may reveal signs of obstruction, but indinavir stones are radiolucent and are usually not visualized on imaging [6]. Similar events have been described with atazanavir [32, 33]. Additionally, many of the antimicrobials used for prophylaxis of opportunistic infections can also exert nephrotoxic effects such as renal tubular acidosis, acute interstitial nephritis (AIN), and acute tubular necrosis (ATN) (Table 13.3). Trimethoprim-sulfamethoxazole can also cause an isolated elevation in serum creatinine without significant change to renal function [6].
Table 13.2
Described renal toxicities of selected antiretroviral medications
Antiretroviral | Renal toxicity |
|---|---|
Tenofovir | Proximal tubulopathy resulting in Fanconi syndrome, acute tubular necrosis, allergic interstitial nephritis, nephrogenic diabetes insipidus |
Atazanavir | Allergic interstitial nephritis (case report), nephrolithiasis |
Indinavir | Allergic interstitial nephritis, intratubular drug precipitation and nephrolithiasis, renal papillary necrosis, renal atrophy |
Stavudine/lamivudine | Renal tubular acidosis, hypophosphatemia (case report) |
Didanosine | Fanconi syndrome, lactic acidosis, nephrogenic diabetes insipidus |
Enfuvirtide | Membranoproliferative glomerulonephritis (case report) |
Table 13.3
Described renal toxicities of selected antimicrobials used to treat opportunistic infections in HIV-positive patients
Antimicrobial | Opportunistic infection | Renal toxicity |
|---|---|---|
Foscarnet | Cytomegalovirus | Acute tubular necrosis, nephrolithiasis and intratubular obstruction, crescenteric glomerulonephritis, nephrogenic diabetes insipidus, renal tubular acidosis |
Trimethoprim-sulfamethoxazole | Pneumocystis jirovecii | Acute tubular necrosis, interstitial nephritis, renal tubular acidosis |
Rifampin | Tuberculosis | Interstitial nephritis, crescenteric glomerulonephritis |
Sulfadiazine | Toxoplasmosis | Nephrolithiasis and intratubular obstruction |
Pentamidine | Pneumocystis jirovecii | Acute tubular necrosis |
Glomerular Diseases Unrelated to HIV Infection
The epidemiology of HIV infection has changed significantly with the advent of effective HIV suppression using HAART. With the improved survival come an increase in the mean age of those living with HIV/AIDS and an increase in the number of comorbidities such as diabetes, hypertension, and atherosclerosis [8, 11, 52]. A review of kidney tissues contained within the Manhattan HIV Brain Bank obtained from patients who had consented to postmortem organ donation for scientific study reveals that nearly one third of 89 deceased individuals had CKD [53]. Notably, while HIVAN was present in some subjects, vascular diseases including arteriolar nephrosclerosis and diabetic nephropathy were also seen, as well as pyelonephritis, interstitial nephritis, fungal infection, and amyloidosis [53]. Other non-HIV glomerular pathology include postinfectious glomerulonephritis, classic FSGS again most commonly seen in African-Americans, and hepatitis B- and C-related glomerulopathies (discussed elsewhere in this chapter) [32].
Treatment of Kidney Disease in HIV
Antiretroviral Therapy in HIVAN
Through the reduction of HIV replication with antiretroviral therapy, the clinical course of HIV infection has improved dramatically since the early 1990s. The goal of HIV therapy is maximal inhibition of HIV replication as measured by consistent plasma HIV RNA (viral load) values below the level of detection [54]. With an undetectable HIV viral load, there should be little circulating free virus and ideally prevent the development [55] or slow the progression of HIVAN [54, 56]. However, it has been shown that in patients who achieved remission of nephritic syndrome in HIVAN, the discontinuation of antiretrovirals results in increased HIV replication and subsequent progression of kidney disease [57, 58]. Additionally, in a study of patients on zidovudine (AZT) therapy with HIVAN comparing renal outcomes in compliant vs. noncompliant patients, subjects who were compliant with AZT treatment experienced a stable creatinine over 8 weeks while their noncompliant counterparts progressed to ESRD requiring dialysis during this same time period [59]. HIV protease inhibitors also provide the same renal-protective benefits; Szczech et al. [56] described a slower decline of kidney function in patients with HIVAN treated with HIV protease inhibitors compared with those not receiving protease inhibitors [56]. These observational studies strongly suggest a therapeutic role for antiretroviral medications in the treatment of HIVAN.
No randomized trials, however, have specifically examined the treatment of kidney disease in HIVAN. One of the few large randomized trials on how to administer HAART that collected renal outcomes was the Strategies for Management of Antiretroviral Therapy (SMART) Trial [60]. SMART randomized 5,472 patients with HIV who had a CD4+ T cell count >350 cells/μL to the continuous use of antiretroviral therapy (2,752 patients; viral suppression) or the episodic use of antiretroviral therapy (2,720 patients; drug conservation) and then followed them for an average of 16 months [60]. Renal events were infrequent in general in the study but slightly more common in the group who had intermittent antiretroviral therapy (drug conservation group) compared with the group who maintained constant HIV suppression (9 of 2,720 patients or 0.2 events/100 person-years vs. 2 of 2,752 patients or 0.1 events/100 person-years, respectively) [60]. Given the consistency of the association between better renal outcomes and HAART among those with HIVAN, clinical practice guidelines currently suggest the initiation of HAART for anyone with HIVAN irrespective of CD4 lymphocyte count [61].
Angiotensin II Blockade in HIVAN
In patients with both diabetic and nondiabetic CKD, antihypertensive agents that affect the renin-angiotensin axis [angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB)] have been proven to reduce proteinuria and slow progression of renal disease [62]. These effects have also specifically been discussed with respect to HIVAN. A retrospective analysis of 18 patients with biopsy-proven HIVAN concluded that AZT and ACEI were independently associated with a slower progression to ESRD [63]. The independent association was confirmed by Wei et al. [64] who described a study where ACEI significantly improved renal survival in HIVAN, even in the absence of HAART therapy. Others have suggested that ACEI therapy is effective in slowing or stabilizing renal disease for up to 12 and 24 weeks in HIV-infected patients with nephrotic and non-nephrotic range proteinuria, respectively [65]. Notwithstanding these findings, others have postulated that ACEIs and ARBs, despite their benefits, are also capable of increasing hypertension and renal disease by increasing the circulating renin leading to potential continued renin-angiotensin-aldosterone system activation [66]. Therefore, it has been suggested more recently that direct renin inhibitors like aliskiren should be considered as an improved alternative to ACEIs and ARBs because they also slow the progression of renal disease without further activation of the renin-angiotensin-aldosterone system [67].
Immunosuppression in HIVAN
Before HAART became available and was documented as the treatment for HIVAN, variable success was reported with the use of corticosteroids in patients with HIVAN. The rationale behind their use lies in the anti-inflammatory effects on the nephron given the prevalence of tubulointerstitial inflammation in HIVAN [68]. Many reported on the use of corticosteroids associated with a reduction in the risk of progression to ESRD [69, 70]. However, relapses after the discontinuation of prednisone were common, often requiring retreatment; additionally, with prednisone came increased incidence of serious complications including avascular necrosis of the femoral head, mycobacterium avium-complex infection, and CMV retinitis [71, 72]. In the era of modern HAART therapy, prednisone does not have a major role in the treatment of HIVAN but can be considered in patients with HIVAN who have refractory nephrotic syndrome despite maximum HAART and ACEI therapy, although no randomized trials exist in the literature to support this [68, 73].
Kidney Transplant
Before HAART, HIV infection was an absolute contraindication for organ transplant, including renal transplant for ESRD. However, with the introduction of HAART, maximal viral suppression, and increased longevity, transplant has become an option for HIV patients with ESRD. Allograft and patient survival after transplant in HIV patients have been shown to near that of non-HIV-infected kidney transplant patients [6]. Eligible patients are those who are on regular HAART therapy for at least 6 months, with undetectable viral loads (<50 copies/μL), CD4+ T cell count >200 cells/mm3, and without AIDS defining illness [6, 74]. Transplant is not without limitations, however, and has been shown to have increased risk of elevated posttransplant creatinine, with acute rejection being more common in HIV transplanted patients [6, 75]. Kidney transplant and antirejection immunosuppression do not increase viral replication and promote progression of HIV disease [75]. However, HIV transplant patients are at greater risk of steroid-resistant rejection, and the metabolism of immunosuppressant medications may be affected by antiretroviral drugs, thereby increasing the risk of inadequate immunosuppression as well as drug-induced toxicity [6, 75]. Nevertheless, transplant still remains a viable option for HIV patients with ESRD who can be followed by a nephrologist and infectious disease transplant teams familiar with the specifics of this special patient population.
Treatment for Glomerular Diseases Other than HIVAN
While approximately half of African Americans with HIV who undergo kidney biopsy have HIVAN, little is known about the treatment of the glomerular diseases that are demonstrated in African Americans without HIVAN and in Caucasians with glomerular diseases. Current evidence does not suggest that HAART benefits patients with HIV-associated immune complex-mediated renal diseases and thrombotic microangiopathies [17, 76, 77]. Among a cohort of 89 persons with HIV undergoing kidney biopsy, the stabilization of kidney function observed in patients with HIVAN concurrent with the use of antiretroviral therapy was not seen among those with glomerular diseases other than HIVAN [11]. While the conclusions from this cohort study are not definitive due to its limitations, one might conjecture that HAART played a role that is augmented by concurrent immunosuppressive therapies to affect patient response to antigen stimulation in the kidney. However, studies of cyclosporine and other agents are small and similarly limited in their ability to draw conclusions [78]. With respect to ACEI and ARB, among persons without HIV infection, a beneficial effect has been demonstrated [79]. However, the effect has not been specifically noted among those with HIV infection [80].
Summary
Renal disease associated with HIV is multifactorial and can affect any part of the nephron, from the well-described direct viral effect causing HIVAN to nephrotoxic effects of antiretroviral drugs. The presence of certain chromosomal foci may increase the risk of developing renal disease among HIV-infected patients of African descent. Due to the current effectiveness of HAART therapy, HIV-infected patients now have improved life expectancies that may lead to the development of chronic kidney disease secondary to both HIV and non-HIV-related causes. Renal biopsy could provide definitive histologic diagnosis but may not be readily available or even necessary for diagnosis and treatment. Appropriate therapy for HIV-related renal disease includes HAART initiation or adjustment and the use of ACEIs and ARBs.
If proteinuria and serum creatinine fail to improve or at least stabilize in the short term after initiation of treatment, renal biopsy should be considered at that time if not previously performed, to further define the renal disease and allow for more aggressive and tailored therapy. Kidney transplant is a viable therapeutic option for end-stage renal disease in HIV patients and is becoming more accepted as HAART can effectively keep the HIV viral load low and CD4+ T cell count high.
Hepatitis B Virus (HBV) Infection
Introduction
Hepatitis B is a DNA virus that is transmitted by infected blood or body fluids including semen and vaginal secretions. The prevalence was increased among hemodialysis patients until the 1970s when the CDC released guidelines for prevention by vaccination of susceptible patients and healthcare workers [81]. The clinical spectrum of HBV infection is variable and includes acute hepatitis, fulminate hepatic failure, an inactive carrier state, chronic hepatitis, cirrhosis, and hepatocellular carcinoma [82]. Extrahepatic disease has been decreasing in incidence with more effective treatment and vaccination for HBV. These extrahepatic manifestations may include renal disease, vasculitides like polyarteritis nodosa, or dermatologic manifestations [83]. Immune complex renal disease represents one of the most common of these extrahepatic manifestations, with membranous glomerulonephropathy representing the major HBV-associated nephropathy (HBVAN) (analogous to HIVAN) [82]. The immune response to hepatitis B infection is correlated with the natural history of the infection and extrahepatic manifestations. Circulating “e-antigen” or HBeAg is a marker of active viral replication. Although normal transaminases can be seen during this phase due to immune tolerance, the patient remains contagious [83]. The immune response to HBV frequently also results in active hepatocyte destruction. With continued immune recognition, the immune system may be able to suppress viral replication without eliminating the virus or virally infected hepatocytes [82]. This situation results in an inactive carrier phase which is manifested as a loss of the HBeAg and development of the anti-HBe antibody [83]. HBV has been more prevalent in renal failure patients, although this association has been decreasing with the improved compliance with HBV vaccination [84]. The presence and absence of HBeAg with treatment of HBV may play an important role in understanding the response of kidney disease to treatment of the virus.
Clinical Presentation and Diagnosis of Kidney Disease in HBV
As with HIV, renal disease associated with HBV commonly presents as nephrotic syndrome. Less common presentations are asymptomatic proteinuria, peripheral edema, hematuria, or rapidly progressive glomerulonephritis (RPGN), which could eventually lead to ESRD [85–87]. The diagnosis is established by serologic evidence of HBV antigens/antibodies, presence of an immune complex glomerulonephritis on kidney biopsy, immunohistochemical localization of 1 or more HBV antigens, and pertinent clinical history, when available [88]. The natural history of HBV-associated kidney disease is not well delineated, and its course may differ depending on the population affected. In children, especially those in sub-Saharan African and Asian countries where HBV is endemic and acquired by horizontal transmission, HBVAN can have a benign course and even achieve spontaneous remission [88]. However, adults would be more likely to develop progressive proteinuria and renal failure, with >50 % of those with nephrotic syndrome and elevated transaminases requiring renal replacement therapy [82]. In developed regions like North America, HBV is less prevalent and largely acquired as a sexually transmitted disease or parenterally by healthcare workers or as a result of intravenous drug abuse [89].
HBV-Associated Nephropathy
The association between hepatitis B infection (HBV) and kidney disease was first reported in the 1970s [90]. It is estimated that one third of the world’s population has had a past or present infection with HBV and approximately 350–400 million people worldwide and 1.25 million people in the US are chronic HBV carriers [83, 91]. The mode of transmission depends largely on the geographical location. In East Asia where HBV is endemic, it is often acquired through vertical transmission from mother to infant and is associated with the chronic carrier state of HBV. Chronic HBV is also endemic in Africa, but transmission is thought to be horizontal with familial clustering, though the precise mechanism of transmission is unknown. In low-prevalence areas like North America, the predominant mode of transmission is sexual or parenteral, with a large majority of cases seen in intravenous drug abusers and those who engage in risky sexual habits (52 % combined) [89]. Hepatitis B infection is the most common cause of secondary glomerulonephritis in endemic regions and occurs more frequently in chronic carriers, since it is uncommon for acute HBV infection in adulthood to become a chronic HBV infection [88, 89]. HBV has a male predilection, with chronic HBV occurring 1.5–2 times more frequently in males vs. females [89]. Efforts to immunize endemic regions for HBV infection have likely played a significant role in lowering the incidence of HBV infections and subsequently HBVAN [89].
Pathogenesis of Kidney Disease in HBV
The exact pathogenesis of HBVAN is not completely understood, but four possible mechanisms have been suggested: (1) direct cytotoxic effect of HBV, (2) immune complex (viral antigen–antibody) deposition, (3) virus-induced immune cell-mediated host response, and (4) virus-induced cytokine-mediated tissue injury [89]. The role of immune recognition and control of the virus, marking the success of treatment, is underscored by the fact that development of anti-HBeAg antibodies and HBeAg clearance are both associated with remission of proteinuria [92, 93]. The HBeAg has been identified as the major antigen found in the subepithelial deposits of HBV membranous nephropathy and thus responsible for the immune complex-mediated injury [89]. Additionally, failure to clear or become immune reactant to HBeAg is associated with progressive loss of kidney function [87, 94, 95].
Types of Kidney Disease in HBV
The glomerular diseases that are associated with chronic hepatitis B infection include immune complex glomerulopathies such as membranous nephropathy [92], membranoproliferative glomerulonephritis (MPGN) [96, 97], focal segmental glomerulosclerosis (FSGS), minimal change disease [98], lupus nephritis [99, 100], IgA nephropathy [101], and polyarteritis nodosa (PAN) [102]. Membranous glomerulonephropathy is the most common HBVAN, and while it typically leads to a progressive nephrotic syndrome in adults, it usually resolves spontaneously in children [89]. On light microscopy, glomeruli are uniformly enlarged with thickened basement membrane and subepithelial spikes of immune complex deposits including C3, IgG, HbeAg, HbcAg, or HbsAG [85, 90]. Membranoproliferative glomerulonephritis appears as thickened capillary walls with segments of double contours and mild to moderate mesangial proliferation [85]. Immune complex deposits consisting mainly of IgG or C3 are deposited in the mesangium and subendothelial space and HBeAg and HBsAg are often implicated [89]. While IgA nephropathy may be a “complication” of HBV infection, it represents one of the most common glomerulonephritides worldwide [103, 104]; thus it can often be an incidental finding on biopsy in regions where both HBV infection and IgA nephropathy are prevalent, such as East Asian countries. Therefore, it can be difficult to ascertain if the primary kidney disease in HBV-infected patients within this population is due to the HBV or the IgA nephropathy [104]. This poses a therapeutic challenge as kidney lesions unrelated to the HBV may not be responsive to traditional anti-HBV treatment.
HBV is also strongly associated with systemic necrotizing vasculitis—polyarteritis nodosa (PAN). The vasculitis affects small to medium sized vessels, and pathology typically shows fibrinoid necrosis and perivascular infiltration [105]. Angiographic lesions typical of PAN, including microaneurysms are frequently observed. PAN due to hepatitis B infection is felt to be caused by immune complex deposition of the hepatitis B virus with excess antigen [89, 106]. Improved immunization compliance worldwide has led to an overall decrease in the frequency of hepatitis B-associated PAN (36–7 %) [106], and in HBV-infected individuals, PAN does not relapse once viral replication has stopped [107].
Treatment
Antiviral Therapy
The goals of treatment of hepatitis B are to sustainably suppress HBV replication and to prevent cirrhosis, hepatic failure, and hepatocellular carcinoma. A number of potential regimens are available for the treatment of adults with HBV all of which are effective in decreasing HBV DNA levels. The choice of a particular treatment strategy should be made based on side effects, medication interactions, presence or absence of HIV coinfection, and the potential for the development of viral resistance. Therapeutic agents can be broadly classified into nucleoside/nucleotide analogs (tenofovir, entecavir, lamivudine, adefovir, and telbivudine) and interferon alpha (conventional interferon-α[alpha]2b and longer-acting peginterferon-α[alpha]2a).
Antiviral agents may be used singly or in combination and vary in required length of therapy (e.g., interferon therapy is administered for a prescribed duration while nucleoside or nucleotide analogs may be used long term) [91]. The decision on which medication to use should be made using a multidisciplinary approach according to a standard Clinical Practice Guideline for HBV infection [83, 91].
Few studies have been performed to assess and compare the efficacy of antiviral agents on HBVAN; in a 2010 meta-analysis to evaluate the efficacy of antiviral or corticosteroid treatment, the remission of proteinuria and clearance of hepatitis B e-antigen (HBeAg) occurred more frequently in the groups receiving antiviral therapy than among those who did not [108].
Nucleoside and Nucleotide Analogs
Nucleoside and nucleotide analogs are oral agents that cause profound HBV DNA suppression. However, the resurgence of HBV DNA levels occurs after the discontinuation of the drugs, potentially leading to the development of resistance, which can be circumvented by long-term antiviral therapy [82]. This class of drugs is renally cleared and therefore requires dose adjustment among persons with decreased renal clearance.
Lamivudine
Lamivudine is a nucleoside analog, the most well studied of the antiviral agents on renal outcomes. Treatment with lamivudine is associated with significant reduction in proteinuria and increase in serum albumin, with reduction of progression to ESRD [109]. Lamivudine is inexpensive, is well tolerated, and is not associated with renal toxicity; however, it is highly susceptible to resistance mutations in hepatitis B [82]. Consequently, current guidelines advise against its use as monotherapy unless other more potent drugs with high barriers to resistance are unavailable; it is acceptable as a second-line agent, often in combination with other antivirals [83].
Tenofovir and Entecavir
As the most potent inhibitors of HBV DNA polymerase with the highest barrier to resistance, tenofovir and entecavir both have grade A1 recommendations for use as a first-line monotherapy in the treatment of HBV [83, 110]. Tenofovir is a nucleotide analog active against lamivudine-resistant HBV and is also active against HIV, making it an appropriate treatment of HIV/HBV coinfected patients (when used in combination with a highly active antiretroviral program for HIV) [111]. While significant data exists on their efficacy in suppressing hepatitis B viral replication resulting in undetectable HBV DNA, loss of HBsAg, HBeAg seroconversion, and normalization of liver enzymes [111], little information exists on their efficacy in the remission of proteinuria in HBVAN. Additionally, tenofovir has been reported to cause renal tubular dysfunction leading to Fanconi syndrome and kidney failure after prolonged use (months to years) in patients taking this drug for HBV or HIV infection [112]. However, tenofovir-related iatrogenic renal impairment is more pronounced in HIV patients, presumably due to the influence of other antiretroviral agents on kidney function [113]. Nevertheless, some have recommended that tenofovir be avoided for these reasons.
Entecavir is a nucleoside analog like lamivudine but is more effective than lamivudine in suppressing viral replication in both HBeAg-positive and HBeAg-negative patients [114]. Cross-resistance has been reported between lamivudine and other nucleoside analogs like entecavir (seen in greater than 7 % of lamivudine-resistant patients); subsequently tenofovir still remains superior to entecavir with regard to resistance barriers [82]. To date, however, no renal toxicities have been reported with entecavir.
Adefovir
Adefovir is a nucleotide analog like tenofovir. Adefovir is more costly than tenofovir and has a higher potential for resistance than tenofovir [83]. Additionally, there is a greater potential for viral rebound after treatment discontinuation [115]. For these reasons, adefovir is not recommended for first-line monotherapy [83]. Finally, the potential for nephrotoxicity should be considered. Renal toxicity is characterized by a tubular nephropathy like that seen with tenofovir, leading to phosphate wasting, osteomalacia, and renal insufficiency [112]. Adefovir was originally FDA approved for HIV therapy at a higher dose, however, was removed from the market at the higher HIV dosing, due to renal impairment.
Telbivudine
Telbivudine is a nucleoside analog that is potent in suppressing HBV replication but associated with a high rate of resistance, making it inferior to tenofovir and entecavir [82, 83]. Given this, it has limited role as a single agent and should be used in combination therapy [116, 117]. The side effect profile includes myopathy and peripheral neuropathy when combined with interferon [82], but no data on nephrotoxicity or its effects on HBV-related glomerular disease are available.
Randomized controlled trials that compare the renal outcomes in patients with HBV-mediated kidney disease undergoing antiviral therapy are lacking; however, the success seen in the observational studies with lamivudine suggests that these regimens, particularly tenofovir and entecavir, will also successfully treat subclinical glomerular disease [83]. Finally, although lamivudine, tenofovir, and entecavir all have activity against HIV; they are contraindicated as monotherapy for HBV in HIV/HBV coinfected patients, since monotherapy for HIV would result in HIV resistance. Rather, in these patients, adefovir, telbivudine, and interferon are more appropriate as HBV monotherapy as these drugs have no activity against HIV and therefore cannot promote resistance [83]. However, current DHHS HIV treatment guidelines recommend starting HAART which includes anti-HBV agents for any HIV/HBV coinfected individual, regardless of the CD4+ T cell count [61].
Interferon (IFN)
IFN is a cytokine produced by white blood cells which has a natural antiviral function by the induction of proliferation of natural killer and CD8+ cytotoxic T cells [89]. Treatment with IFN is designed to have a finite duration; usually at least 48 weeks is recommended [82, 83, 89]. IFN potentially offers curative treatment with the goal being immune-mediated viral control and sustained suppression after treatment is concluded [82, 83]. Additionally, Interferon does not cause resistance as may be the case with nucleoside and nucleotide analogs [82, 83]. Pegylated-IFN alone or in combination with lamivudine is more effective than lamivudine alone at suppressing HBV DNA and inducing HBeAg seroconversion which is the ultimate goal of HBV treatment and is clearly associated with positive long-term outcomes [118, 119]. Treatment with IFN has been shown to suppress proteinuria and improve renal function in patients with HBVAN [120, 121]. The disadvantages of IFN treatment include the inconvenient and/or uncomfortable subcutaneous injections and frequent side effects including flu-like symptoms, increased rates of infection, and gastrointestinal effects [82]. IFN is contraindicated in patients with decompensated HBV cirrhosis, autoimmune disease, uncontrolled depression or psychosis, and in pregnancy [83]. Although combination IFN/telbivudine is highly effective at HBV suppression, it is not recommended due to the risk of severe peripheral neuropathy [83].
Immunosuppressive Therapy in HBVAN
Corticosteroid therapy is the treatment of choice for idiopathic nephrotic syndrome, but this benefit does not extend to nephrotic syndrome secondary to HBVAN [108]. In fact, the treatment of HBVAN with corticosteroids has been shown to cause an increase in HBV DNA replication, increase in HBeAg, rising transaminases, persistence of proteinuria, and worsening renal function [82, 83, 89]. Renal biopsies in patients with HBVAN obtained before and after corticosteroid therapy have demonstrated the progression of glomerulosclerosis on light microscopy and virus-like particles within the glomeruli on electron microscopy in tissue after corticosteroid therapy, thus confirming that corticosteroid therapy contributes to the progression of renal disease in HBV [122]. Other immunosuppressive agents like rituximab are generally not recommended for treatment of HBV or its associated renal diseases, as they generally have similar effects to corticosteroids in reactivation of HBV replication and worsening HBVAN nephritic syndrome [82, 83]. In patients who must receive these immunosuppressive agents for other comorbidities, prophylaxis with lamivudine or entecavir has been shown to be helpful in significantly reducing the reactivation of HBV replication and subsequently reducing mortality [82, 83, 110].
Transplant
Although renal transplant is a useful option to ESRD, the concern in patients with HBV infection is that of the profound immunosuppression mediated by the antirejection agents including corticosteroids. In the absence of antiviral treatment in the posttransplant period, there is a higher risk of progression of HBV and increased incidence of hepatocellular carcinoma [123]. As expected, in the presence of adequate antiviral therapy, patient and graft survival are superior to non-treated patients [123]. The only caveat is that IFN therapy, which can be very useful in non-transplant patients, is contraindicated after transplant as it has been found to have direct nephrotoxic effects on the graft and precipitate acute graft rejection [123]. Additionally, despite the nephrotoxic effects of some nucleotide/nucleoside analogs, therapy with these drugs has been shown to be beneficial in renal transplant patients in treating and preventing HBVAN; one study has even demonstrated 100 %, 97.6 %, and 88 % graft survival at 5, 10, and 20 years, respectively, in patients treated with the nucleoside and nucleotide analogs, with no graft loss related to either drug nephrotoxicity or HBVAN [123]. However, despite these promising results, frequent surveillance of renal clearance parameters is necessary to monitor for HBVAN progression posttransplant.
Prevention Strategies
The transmission of HBV has already been described earlier in this chapter. Strict screening practices at blood banks and hemodialysis centers reduce parenteral transmission, and the administration of HBV immune globulin and HBV vaccine to newborns of HBV carriers reduces vertical transmission [110]. Safer sex practices will reduce the numbers of sexually transmitted HBV, however, HBV vaccination remains the crux of HBV prevention. Increased compliance to vaccination schedules has reduced the incidence and prevalence of HBV worldwide, thereby reducing the incidence and prevalence of HBVAN and other HBV-related diseases [89].
Summary
The improvement in efficacy and availability of treatments for HBV has significantly improved the outcome of HBV-infected patients. There is a paucity of studies examining the effect of these therapies on HBVAN. The limited data however suggest that the remission of proteinuria and improvement in renal clearance occur among those who clear the HBeAg and may be more related to the success of therapy in this clearance than in the therapy chosen. In a manner similar to the disappearance of endocarditis-related glomerulonephritis after the introduction of antibiotics, the incidence and prevalence of HBV-associated glomerular diseases may eventually regress with increasingly effective and available treatments. Current available therapies have advantages and disadvantages. The recommended first-line therapy for HBV treatment is monotherapy with tenofovir or entecavir; however, combination therapy or therapy with interferon is also available. Special populations may have exceptions (i.e., renal transplant patients, pregnant patients, HIV-coinfected patients). Nephrotoxicity is a common side effect among some of the nucleoside and nucleotide analogs, but this does not prohibit their use especially in patients with renal insufficiency; the doses must simply be adjusted for renal function. Comanagement of the HBVAN with a nephrologist will always be appropriate in light of the known challenges associated with treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







