Fig. 1
The pseudo-kidney sign. The strong echogenic center (corresponding to the lumen content) is surrounded by an echo-poor rim (thickened bowel wall) in a case of colonic adenocarcinoma
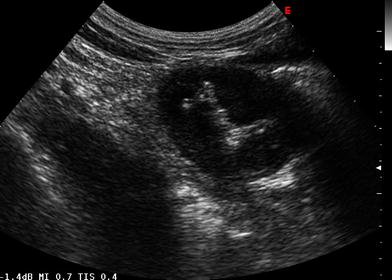
Fig. 2
The “target like” pattern in a case of small bowel lymphoma
Since 1980, many authors have reported the possibility to reach the diagnosis of gastrointestinal neoplasia by using a US-guided FNB (Abbitt 1991; Allen and Irwin 1997; Ballo and Guy 2001; Bhaduri et al. 1999; Bree et al. 1991; Carson et al. 1998, Das and Pant 1994; Dodd et al. 1998; Marco-Domenech et al. 2001; Farmer et al. 2000; Green et al. 1988; Heriot et al. 1998; Ho et al. 2003; Javid et al. 1999; Ledermann et al. 2001; Shidham et al. 1998; Solbiati et al. 1986; Torp-Pedersen et al. 1984; Tudor et al. 1999). Sonographically guided fine needle aspiration biopsy of a bowel wall lesion was first performed in 1981 by Ennis and Mac Erlean (1981).
2 Indications and Contraindications
Percutaneous US-guided FNB of a gastrointestinal mass is performed mainly when:
1.
The lesion is well visualized at US study;
2.
The lesion is endoscopically inaccessible (i.e., localized between the ligament of Treitz and the ileocecal valve);
3.
The lesion is situated in the submucosa (as frequently seen in gastrointestinal lymphoma), is intramural or develops in the subserosa (i.e., in gastrointestinal stromal tumors), and is thus not accessible by conventional endoscopic biopsy;
4.
A previous biopsy during endoscopy or EUS is non-diagnostic or only necrotic material is obtained;
5.
Endoscopy cannot be performed for uncooperative patients or for contraindications;
6.
It is impossible to obtain an adequate biopsy sample during endoscopy due to presence of severe stenosis.
Contraindications are represented only by a severe coagulative impairment (i.e., platelet count ≤40000/mm³; prothrombin time ≤40 %) and by distension of bowel loops.
3 Technique
Percutaneous FNBs are frequently performed under US guidance with a real-time convex transducer (3.5–5 MHz) by both a “free hand” technique and a biopsy-guide attachment. Fine needles (i.e., needles with an outer diameter <1 mm-20 gauge) of both the “cutting” or “non-cutting” type, are frequently used to obtain a cytological and/or histological diagnosis (Ballo and Guy 2001; Carson et al. 1998; Marco-Domenech et al. 2001; Ennis and Mac Erlean 1981; Green et al. 1988; Javid et al. 1999; Ledermann et al. 2001; Solbiati et al. 1986; Torp-Pedersen et al. 1984). Some authors have also used large (1.2 mm-18 gauge) cutting needles (Ballo and Guy 2001; Carson et al. 1998; Marco-Domenech et al. 2001; Farmer et al. 2000; Ledermann et al. 2001; Tudor et al. 1999). The use of large cutting needles has been experimentally demonstrated to be safe (Akan et al. 1998) and not associated to a mortality and/or morbidity increase (Marco-Domenech et al. 2001; Tudor et al. 1999). Patients must fast from the night prior to the biopsy and usually bowel preparation or antibiotic prophylaxis are not needed (Tudor et al. 1999). All patients sign a written informed consent. The procedure is usually performed on both an inpatient/outpatient basis (Ballo and Guy 2001) and often without local anaesthesia; however, some authors utilize local anaesthesia by infiltration of 5–10 ml of 1 % lidocaine hydrochloride into the abdominal wall by means of a 25-gauge needle (Ho et al. 2003; Tombesi et al. 2011). In many cases, color and power Doppler are useful to evaluate and avoid large vessels along the needle tract (Fig. 3) (Marco-Domenech et al. 2001; Ho et al. 2003). Briefly, percutaneous FNB is frequently performed by two operators, one holding the transducer and the second manipulating the needle. In “pseudokidney” lesions, it is important to place the needle in the hypoechoic rim, thus avoiding traversing of the echogenic center (Figs. 4, 5) which corresponds to the mucosa and the lumen of gastrointestinal loop. When a “non-cutting” needle is used, it is fitted to a 10–20 ml syringe attached to an aspiration piston, and is monitored while advancing in the lesion. When the tip of the needle (shown on the scan) is in the tumor wall, the piston is completely retracted and the needle is moved back and forth three or four times in the tumor (Torp-Pedersen et al. 1984).
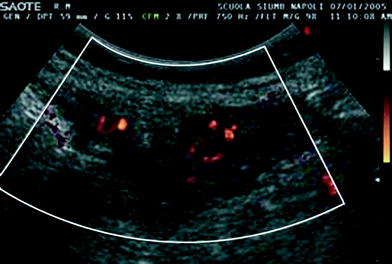
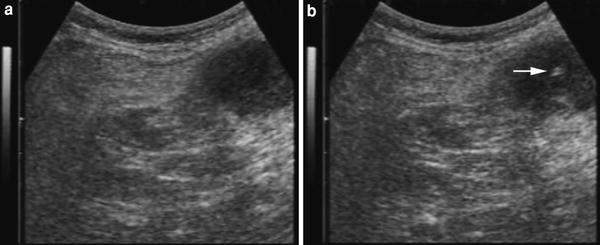
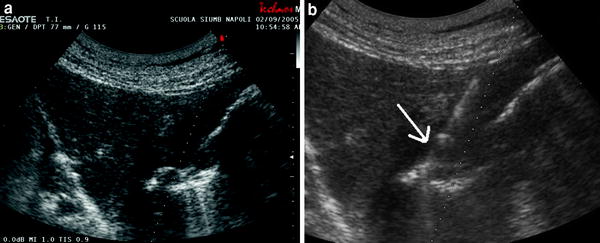

Fig. 3
Power Doppler of a diffuse thickened gastric wall shows clear vascular signals (to be avoided during biopsy)

Fig. 4
a Transverse sonogram of abdomen showing gastric hypoechoic wall thickening. b Ultrasound-guided fine needle aspiration biopsy (non-cutting needle) of the lesion (arrow indicates the tip of the needle): cytological diagnosis of gastric adenocarcinoma

Fig. 5
a A diffuse thickening of the gastric wall (endoscopic biopsy resulted not diagnostic). b Ultrasound-guided needle biopsy with a cutting needle. The needle is well-visualized (arrow): histological diagnosis of undifferentiated carcinoma
When aspiration is completed, the negative pressure is equilibrated and the needle is withdrawn. After withdrawal of the needle, the syringe is disconnected, filled with air and reconnected and the material in the needle is expelled onto a glass slide and smeared. The specimen are then air-dried and fixed in ethanol; Papanicolau and May-Grunwald–Giemsa are the standard staining techniques used, while if a carcinoid tumor is suspected the Grimelius staining method is used (Solbiati et al. 1986).
When a “cutting-type” needle is used, the needle is advanced until lesion indentation is seen, then the trigger mechanism is fired and the needle is withdrawn, obtaining a histological sample (Fig. 5; Tudor et al. 1999).
Core-needle biopsy samples are fixed in formalin and embedded in paraffin wax; 3–4 μm-thick sections are cut and then stained with hematoxylin/eosin. Further immunohistochemical analysis of the material is then possible for specific indications requested. In all cases, the presence of a cytopathologist, to assess specimen adequacy during the procedure, would result in a high diagnostic outcome (Ballo and Guy 2001); however, if this is not possible, two needle punctures during the first session should be performed.
After the procedure is completed the outpatients are observed for 2 to 4 h in order to monitor eventual immediate complications such as persistent pain, fever, or bleeding at puncture site. Some authors prefer to perform an ultrasound control before discharging patients. In our Department we performed FNBs of gastrointestinal masses, with ultrasound guidance, by using a 3.5–5 MHz convex probe equipped with a biopsy device. All the procedures were performed in outpatients with no general or local anaesthesia and we used both large cutting and fine non-cutting needles (particularly in suspected lymphoma lesions in which we perform both a biopsy and cytofluorometry).
Ultrasound-guided FNBs (Ledermann et al. 2001) have been performed mostly in suspected primary or metastatic neoplastic gastrointestinal lesions, also in those patients with HIV infection (Bhaduri et al. 1999) in which gastrointestinal manifestations (such as opportunistic infections or lymphoproliferative or neoplastic disease) occur in about 50 % of the patients and endoscopic diagnosis is not possible due to the submucosal nature of the disease (Fig. 6).
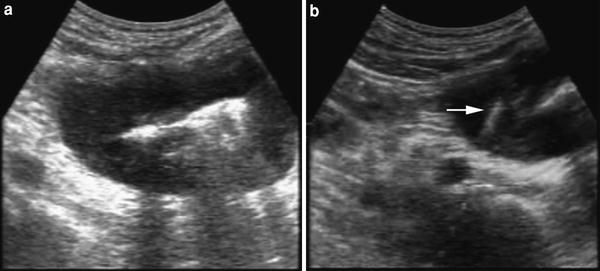

Fig. 6
a Small bowel wall involvement. b Ultrasound-guided fine needle biopsy of bowel wall involvement. The needle tip (arrow) is visible in the hypoechoic wall. Final diagnosis: small bowel lymphoma
4 Results
Table 1 summarizes the most important studies published on the use of FNBs of gastrointestinal wall lesions. Because of the relatively small population, and the absence of a clear gold standard in order to assess the true-negative and false-negative results, few data are available regarding sensitivity, specificity, and overall diagnostic accuracy of the procedure. When the data are reported, sensitivity, specificity, diagnostic accuracy, predictive positive value and predictive negative value are 90–91, 100 (no false-positive results), 80–100, 100, and 67 % respectively, thus equal to those obtained in other malignant abdominal diseases (Ballo and Guy 2001; Javid et al. 1999; Torp-Pedersen et al. 1984).
Table 1
Published studies on the use of percutaneous FNBs of gastrointestinal wall lesions
Reference | Number | Site | Needle type | Gauge | Complications |
|---|---|---|---|---|---|
Ennis and Macerlean (1981) | 7 | B | NC | No | |
Torp-Pedersen et al. (1984)a | 78 | S-SB-C | NC
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





