Fig. 3.1
Cardia in a normal child (alcian blue PAS at pH 2.5). Notice that the surface columnar cells have a mixed staining pattern of magenta and blue indicating presence of neutral and acid mucins
Pancreatic acinar tissue occurs, probably as a developmental ectopia, at the cardia or the antrum in the stomach of children (Wang et al. 1996; Integlia et al. 1997; Popiolek et al. 2000) (Fig. 3.2) . Sixteen percent of 69 children studied were affected at the gastroesophageal junction (Popiolek et al. 2000). In contrast to adults, in children, pancreatic acinar metaplasia is usually seen in a background of uninflamed gastric mucosa.
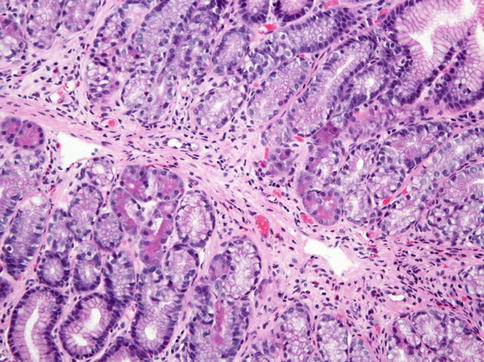

Fig. 3.2
Pancreatic acinar metaplasia. Pancreatic acinar cells with granular, eosinophilic cytoplasm are seen in the basal portion of some glands in this biopsy sample of the antrum
The gastric body has thick mucosal folds or rugae and areae gastricae, the furrows between the undulations. We refer to the fundus as that part of the body (corpus) that bulges above and to the side of the cardia. The mucosa is surfaced by neutral mucin-containing columnar cells, as is all the stomach, with a more flat architecture than the more villiform cardia and antrum. The pits or foveolae comprise about one-quarter or less of the thickness and overlie the glandular zone containing parietal and chief cells.
The antrum takes up the distal one-third of the stomach, ending at the pyloric junction. The proximal boundary is represented by a line from the incisura angularis on the lesser curve to the opposite wall. The foveolar zone occupies up to 50 % of the mucosa and the underlying glands are mucous producing. Histologic boundaries do not precisely follow the anatomic definitions (Owen 2007).
The inflammatory cell population in the normal childhood stomach is not clearly defined. Only a few studies have attempted to provide quantitative data for mononuclear inflammatory cells and eosinophilic granulocytes (Table 3.1). In general the pediatric normal stomach is thought to have a sparse number of inflammatory cells comprised of scattered individual, not clustered, lymphocytes, plasma cells, and, rarely, eosinophils (Lash et al. 2009). In the antrum and fundus, eosinophils are usually present immediately above and also within the muscularis mucosae and not near the luminal surface. Furthermore, eosinophils are not seen within the surface epithelium and only rarely within the glandular epithelium (Lowichik and Weinberg 1996; DeBrosse et al. 2006). Aggregates of lymphocytes, small and without germinal centers, occur infrequently and usually only in the deep mucosa (Carpentieri et al. 2000). Although we are unaware of studies evaluating the inflammatory cell content of the cardia in normal children, it is our impression that this zone, like other major mucosal junctions, normally contains lymphocytes, some in aggregates and a few plasma cells. If confirmed, this information is very important in view of the considerable attention being paid to carditis in adult patients.
Table 3.1
Inflammatory cell population counts in “normal” children
Stomach layer | Jevona | Ashornb | De Giacomoc | DeBrossed | Lowichike |
|---|---|---|---|---|---|
Antrum lamina propria | |||||
Lymphocytes/mm2 | 258 (225–292) | 604 (414–793) | |||
Plasma cells/mm2 | 197 (159–236) | 411 (274–548) | |||
Eosinophils/mm2 | 4 (4–13) | ||||
Eosinophils/hpf | 1.9 ± 1.3 | <10 | |||
Body lamina propria | |||||
Lymphocytes/mm2 | 321 (262–381) | 590 (437–743) | |||
Plasma cells/mm2 | 211 (172–249) | 641 (479–803) | |||
Eosinophils/mm2 | 41 (13–96) | ||||
Neutrophils/mm2 | 8 (8–24) | ||||
Eosinophils/hpf | 2.1 ± 2.4 | <10 | |||
Antrum surface epithelium | |||||
Lymphocytes/100 cells | 3.5 (2–6) | 5.15 (±1.95) | |||
Eosinophils/hpf | 0.0 ± 0.0 | ||||
Body surface epithelium | |||||
Lymphocytes/100 cells | 3.8 (2–7) | ||||
Eosinophils/hpf | 0.0 ± 0.0 | ||||
3.2 Professional and Technical Matters
Needless to say the endoscopist must be technically skilled and familiar with the anatomy of the normal and abnormal pediatric stomach. Biopsy in most instances is necessary for proper interpretation of gastropathies. Commonly the gross mucosal appearance does not match the histologic one, making inaccurate conclusions based on gross observation alone (Black et al. 1988; Carpenter and Talley 1995) (Table 3.2). In children, gastritis remains under-recognized and poorly characterized because of the flawed tendency to rely on macroscopic appearances at endoscopy and an apparent reluctance to perform multiple gastric biopsies (Dohil et al. 1999). Exceptions such as stress-induced erosions can be adequately evaluated by gross inspection. Given the frequent discord between gross and microscopic findings, the endoscopist should sensibly describe observations with simple terms devoid of diagnostic inferences; for example, it is more appropriate to call mucosa erythematous or red rather than inflamed or gastritis, which is a histopathologic judgment.
Table 3.2
Patterns of gastritis
Pathologic diagnosis | Etiologic agents | Pathologic findings | Endoscopic findings |
|---|---|---|---|
Acute suppurative gastritis | Acute H. pylori, streptococcal and other bacteria | Neutrophilic inflammation | Normal, mucosal swelling, erosions |
Acute erosive gastropathy | NSAID, other drugs, stress | Erosions, ischemic lesions, minimal inflammation | Erosions, superficial hemorrhages |
Chronic and chronic active gastritis | Chronic H. pylori, autoimmune | Lymphoplasma, neutrophilic infiltrate, epithelial damage | Normal, erythema, antral nodularity, ulcers |
Lymphocytic gastritis | Hypersensitivity to gliadin, others | Chronic active inflammation, intraepithelial lymphocytes | Varioliform |
Granulomatous gastritis | Crohn’s disease, chronic granulomatous disease, other. | Focal chronic active inflammation with granulomas | Thickened folds, narrowed antral outlet, ulceration |
Eosinophilic gastritis | Food allergy, drug allergy, parasitic | Abundant eosinophils in lamina propria and epithelium | Normal, prominent folds, nodules |
Chronic reactive gastropathy | NSAID, bile reflux | Foveolar hyperplasia, ectasia, edema, muscle extension | Erosions, erythema |
Congestive gastropathy | Portal hypertension | Foveolar hyperplasia, vascular ectasia, with/without microthrombi, no inflammation | Hemorrhages, antral red spots, snakeskin appearance in body |
Reactive gastropathy with features suggestive of radiation/chemotherapy effect | Radiation, chemotherapy | Foveolar hyperplasia with glandular epithelial atypia, little inflammation | Ulceration |
Hypertrophic gastropathy | Classic Ménétrier’s disease, CMV, H. pylori, lymphoma | Massive foveolar hyperplasia (classic Ménétrier’s disease) | Giant gastric folds in body, sometimes erosions |
The endoscopist must provide a non-biased gross description and location for each biopsy. Although a biopsy protocol was recommended by the updated Sidney System (Dixon et al. 1996), gastroenterologists do not often follow such a rigorous sampling protocol (Lash et al. 2009). The number of biopsies at sites depends on gross findings, but we recommend at least two from each site. These biopsies are then immersed in formalin in properly labeled containers. Usually we do not exceed three biopsies per cassette in order to obtain representative sections from each piece. Processed biopsies are embedded with care to ensure proper cross-sectional orientation. We prepare two slides per paraffin block cut at 5 μm with two ribbons containing five to eight sections per slide. Hematoxylin and eosin (H&E) staining is routine. Helicobacter pylori organisms can be seen in H&E-stained biopsy samples. However, in up to 30 % of cases a more sensitive stain, such as Steiner, Giemsa or Diff-Quik, Gram, or Genta, may be needed (Genta et al. 1994b; El-Zimaity et al. 1998; Rotimi et al. 2000). Eshun et al. (2001) claim immunohistochemical methods identify H. pylori definitively and more often than silver stains or the rapid urease test. The rapid urease test is insufficiently reliable to use alone, without histologic methods (Madani et al. 2000).
In reality there are two diagnostic specialists, one being the endoscopist functioning as the morbid anatomist and the microscopist being the histopathologist. A common language is required for the two experts to arrive at the proper diagnosis. Although the revised Sydney System (Dixon et al. 1996) was developed to provide pathologists with guidelines for generating uniform diagnostic reports, its usefulness to pediatric endoscopists is limited, because its major focus is grading the severity of chronic gastritis, atrophy, and intestinal metaplasia, which are of concern more in adults than children (Dohil et al. 1999). As recognized in the updated Sidney System, “acute” gastritis is seldom seen in biopsies as it refers to either the initial phase of H. pylori infection, acute bacterial suppurative gastritis, or acute hemorrhagic or erosive gastritis, which is usually related to acute chemical or irritant injury (Dixon et al. 1996). The vast majority of inflammatory processes seen on biopsies are chronic in nature, and they may or may not include an element of neutrophilic “activity.” When present, neutrophilic infiltration in itself should not be interpreted as evidence of “acute” gastritis but should be referred in the diagnosis as chronic “active” gastritis, which implies the presence of neutrophils in a background of chronic inflammation. The spectrum of causes of gastritis differs in children than in adults. A sample of the experience of Dimmick et al. (1997) is presented in Table 3.3. The same authors proposed an etiopathogenetic classification that illustrates the spectrum of gastritis in childhood (Table 3.4).
Gastritis type | N (%) |
|---|---|
Helicobacter pylori gastritis | 69 (19.2 %) |
Crohn’s disease | 67 (18.6 %) |
Eosinophilic gastritis | 8 (2.2 %) |
Celiac disease | 6 (1.6 %) |
Chronic granulomatous disease | 3 (0.8 %) |
Autoimmune gastritis | 3 (0.8 %) |
Henoch-Schonlein’s disease | 2 (0.3 %) |
Helicobacter heilmannii gastritis | 1 (0.3 %) |
Graft-versus-host disease | 1 (0.3 %) |
Chronic nonspecific gastritis | 200 (55.5 %) |
Table 3.4
Pediatric gastritis/gastropathy: etiopathogenetic spectrum
Infectious | Helicobacter pylori |
Helicobacter heilmannii | |
Other bacteria | |
Streptococcus | |
Staphylococcus | |
Mycobacterium tuberculosis | |
Treponema pallidum | |
Fungi | |
Candida albicans | |
Parasites | |
Anisakis simplex | |
Giardia lamblia | |
Virus | |
Cytomegalovirus | |
Herpes simplex | |
Noninfectious immune | Celiac disease |
Graft-versus-host disease | |
Eosinophilic gastritis | |
Autoimmune gastritis | |
Henoch-Schonlein’s disease | |
Polyarteritis nodosa | |
Genetic/metabolic disorders | Cobalamin C disease |
Chronic granulomatous disease | |
Chemical/toxin injury | NSAIDs |
Other drugs | |
Bile reflux | |
Physical agent injury | Tubes |
Radiation | |
Vascular | Portal hypertension |
Unknown/uncertain | Crohn’s disease |
Ulcerative colitis | |
Ménétrier’s disease (not associated with CMV infection) | |
Chronic gastritis not otherwise specified etiologically |
3.3 Gastropathies
This review focuses especially on the gastric inflammatory disorders of childhood. Other currently relevant gastropathies are included. We do not address in-depth neoplastic entities.
3.3.1 Infectious Gastritides
3.3.1.1 Helicobacter pylori Gastritis
Helicobacter pylori, now accepted as a very common cause of gastritis in adults and children, is the foremost infectious etiology of gastric inflammation. H. pylori has a predilection for gastric surface (foveolar) epithelium, whether that is in the stomach or the metaplastic gastric epithelium of duodenum and Barrett’s esophagus. We and others (Oguzkurt et al. 2001) have seen H. pylori on foveolar epithelium of ectopic gastric mucosa in Meckel’s diverticulum.
H. pylori is established as an important cause of peptic ulcer disease, atrophic gastritis, intestinal metaplasia, gastric carcinoma (usually the intestinal type and occasionally the diffuse form), MALT (mucosa-associated lymphoid tissue) lymphoma of the stomach, and rarely T-cell lymphoma; evidence is emerging suggesting a role in autoimmune gastritis and B12-deficiency anemia (Faller et al. 1998; Kaptan et al. 2000; D’Elios et al. 2004). In addition, over the past two decades, an association between H. pylori and pediatric iron-deficiency anemia has been established (Baysoy et al. 2004; Pacifico et al. 2010). However, the issues of whether H. pylori infection is linked causally to iron-deficiency anemia in children and whether treatment or resolution of H. pylori infection would improve iron stores or resolve iron-deficiency anemia in children are still matters of great debate (Pacifico et al. 2010).
H. pylori infection is acquired early in life, usually before the age of 10 years, and in the absence of antibiotic therapy, it generally persists for life (Sherman 2004; Pacifico et al. 2010). Given the prominence of H. pylori infection worldwide and the multitude of consequences, including the probability that in some individuals no disease is caused or at least not yet recognized, the pressing dilemma presently is therapeutic – who requires treatment? (Sherman 2004; Kato and Sherman 2005; Buonavolonta et al. 2011). Based on an extensive literature review, Imrie et al. suggest there is no reason to treat all infected children for the purposes of cancer prevention (Imrie et al. 2001). In support of this recommendation, Nardone et al. (2001) found no alteration in markers of genomic instability that usually precede dysplasia and neoplasia. Recent recommendations by NASPGHAN and the American College of Gastroenterology continue to support this approach (Chey and Wong 2007; Koletzko et al. 2011). Treatment of those with H. pylori gastritis and ulcers is supported without question. However, controversy continues regarding therapy for non-ulcer dyspepsia (Pacifico et al. 2010). Diagnostic testing for H. pylori infection is not recommended in children with functional abdominal pain according to current NASPGHAN guidelines (Koletzko et al. 2011). Current recommendations do not approve a “test and treat” approach but to select a patient in whom organic disease is expected based on detailed medical history and physical examination.
Enthusiasm for explaining and treating all peptic ulcer disease as an infection-based disorder subsided with recognition that ulcer disease in children (and adults) does occur without H. pylori infection (Dohil and Hassall 2000; Stringer et al. 2000). In one study, about 20 % of ulcer disease in children is unrelated to H. pylori gastritis (Hassall et al. 2002). Of 43 patients with duodenal ulcers, 9 were H. pylori negative. The cause was unknown; there was no association with NSAID use, Crohn’s disease, or Zollinger-Ellison syndrome.
In the gastrointestinal tract, the mucosal defense to infections is usually secretory IgA that tends to attenuate inflammation and injury of the mucosa. In contrast, the reaction to H. pylori appears to be preferentially cell mediated (Ernst et al. 1997). H. pylori through adherence and injury to gastric epithelium induces cytokine production that triggers the immune system. T helper 2 (Th2) lymphocytes are minimally evoked, whereas Th1 are stimulated (Luzza et al. 2001a; Shimizu et al. 2004; Pellicano et al. 2007). These lymphocytes presumably arrive from elsewhere in the intestine or from the minimally present lymphoid tissue in the stomach. The Th1 cells promote inflammation and can induce autoantibody formation and cell-mediated epithelial injury and apoptosis. The outcome of this form of immune response to H. pylori infection is persistent chronic active inflammation, epithelial injury, and, if prolonged, atrophy, intestinal metaplasia, and neoplasia (adenocarcinoma and lymphoma).
Ulcer formation in the stomach or duodenum may be due to exposure to inflammatory mediators bathing the gastric mucosa and washing down into the duodenum. Warren (2000) views duodenal ulcers as distally occurring gastric ulcers in the circumstance of H. pylori gastritis. Does gastric metaplasia of the duodenum bring the organisms closer and therefore increase the incidence of ulceration in the duodenum? A review of gastric metaplasia in children by Hiruki et al. (1993) suggests that this may be the case. In this study, 12 children with H. pylori gastritis and duodenal ulcer, 15 with H. pylori gastritis without ulcer, and 23 with normal stomachs were examined. Metaplasia was found in 10 of the 12 with ulcers, in 3 of the 15 without ulcers, and in 5 of 23 normals. H. pylori was found on metaplastic epithelium in four with ulcers and in none without ulcers.
H. pylori genotyping reveals multiple types which with host variables probably explain the diversity of consequences of infection (Nogueira et al. 2001) . The cytotoxin-associated gene (cagA), a vacuolating cytotoxin (VAC), and others are now being correlated with degree of gastritis and outcome. More severe pathologic change and more ulceration occur with cagA- and VAC-positive strains (Alarcon et al. 2000; Yahav et al. 2000; Luzza et al. 2002). The pathogenesis of H. pylori gastritis continues to be studied, and recent reviews are available in the literature (Alarcon Martinez-Gomez et al. 2013; Cid et al. 2013).
H. pylori causes acute and chronic active gastritis and slowly regressing chronic inflammation after successful antibacterial therapy. Patients are seldom biopsied during the short-lived acute phase of H. pylori infection. Knowledge of the pathologic aspects of the acute phase is limited to a few case reports (Rocha et al. 1991; Sobala et al. 1991; Mitchell et al. 1992). In one such a case, an infant who presented with vomiting and hematemesis, endoscopy showed antral erosions and, microscopically, neutrophilic inflammation of the columnar epithelium on the surface and in foveolae (Mitchell et al. 1992).
Endoscopically, the mucosa in chronic active H. pylori gastritis may be normal or erythematous; particularly in children the antrum is nodular (Fig. 3.3a). In children, the chronic active form is a pangastritis with the antrum most heavily inflamed, the body the least, and the cardia intermediate in intensity (Hassall and Dimmick 1991). Mucosal nodules are created by hyperplastic lymphoid follicles and are a strong indicator of H. pylori infection and of a high grade of inflammation (Carpentieri et al. 2000; Luzza et al. 2001b) (Fig. 3.3b). With prominent germinal centers, these nodules can span the thickness of the mucosa. It is suggested that in contrast to adult H. pylori gastritis, the pediatric stomach reacts with less neutrophilic inflammation and with more lymphoid stimulation (Riddell 1999; Whitney et al. 2000). Focal gastritis does occur usually in the antrum, without involvement of the body and cardia. To what extent this pattern represents effects of partial treatment is unknown. Hypertrophic gastropathy due to H. pylori gastritis is a rare manifestation in children (Tokuhara et al. 2007; Iwama et al. 2010; Yoo et al. 2013); one case with protein-losing gastropathy has been reported (Yamada et al. 1997).
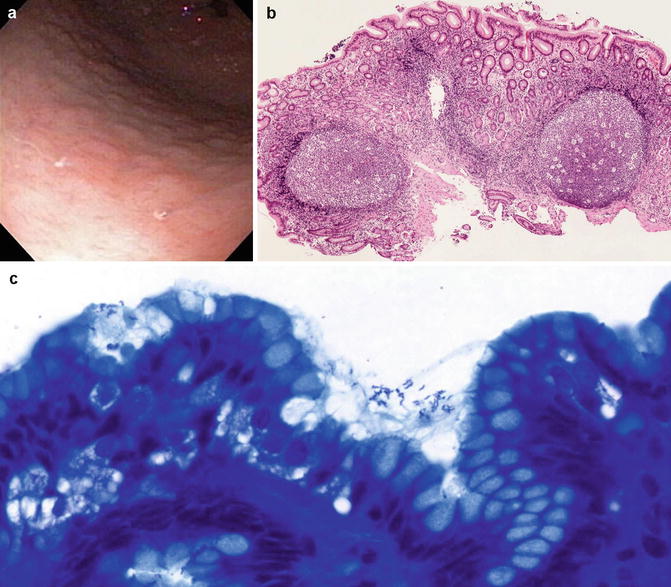

Fig. 3.3
H. pylori gastritis. (a) Endoscopic view of the antrum. Notice the multiple mucosal nodules. (b) Biopsy of nodular antrum showing pan-mucosal inflammation, more intense superficially, and lymphoid hyperplasia. (c) H. pylori organisms are short and bent in the middle in this Diff-Quik-stained biopsy. They are present on the cell surfaces and in the mucus
Microscopically, H. pylori are located especially on the mucosal surface and lumen of the upper part of the pit. They are small, slightly curved, and identifiable in H&E-stained slides or made more obvious by silver or other stains (Fig. 3.3c). The presence of organisms is almost always accompanied by neutrophils, but these are mostly located in the lamina propria and at the neck of the pit. The infection induces epithelial injury, which is evidenced by distortion of the gastric surface epithelium that changes from an orderly picket fence to a cobblestone pattern as the cells become more amoeboid in appearance (Warren 2000). Mucin production is reduced. Ultrastructurally, the normal cells with a microvillus-covered surface have cytoplasmic fibrils anchoring the villi to the cell base. Subjected to the effects of H. pylori attachment, the cell microvilli are lost as are the cytoplasmic fibrils, and consequently the cell rounds up.
Studies of patients followed after successful organism eradication are interesting (Rauws et al. 1988; Genta et al. 1993; Forbes et al. 1996; Tepes et al. 1999; Warren 2000). Neutrophils disappear quickly; Tepes et al. (1999) found absence of neutrophils after 2 months, and in our experience the organism disappears synchronously with the neutrophils. Chronic inflammation subsides much more slowly, up to 4–7 years, and lymphoid aggregates may remain (Warren 2000). Epithelium and mucin production return to normal; metaplasia remains and atrophy, according to some, diminishes (Tepes et al. 1999). Genta (1997) cautions that atrophy might be overcalled in the face of extensive inflammation of the lamina propria displacing rather than destroying glands. H. pylori gastritis patients with gastroesophageal reflux treated with acid suppression show a shift of the pattern of gastritis. The gastritis becomes body dominant, rather than antral, and they may develop atrophy (Voutilainen et al. 1999).
Chronic carditis is a subject of interest in adults because of concern for intestinal metaplasia and adenocarcinoma in the cardia. Not surprisingly, biopsies of the cardia reveal H. pylori as a common cause of carditis (Genta et al. 1994a; Voutilainen et al. 1999; Morini et al. 2001; Odze 2005).
Adenocarcinoma and lymphoma of the stomach arise as a consequence of H. pylori chronic infection. Lymphomas are most often MALT-related and may retreat with antibiotic elimination of the infection (Hussell et al. 1993; Wotherspoon et al. 1993; Graham 1994; Endo et al. 1995; Kuipers et al. 1995; Roggero et al. 1995; Uemura et al. 2001).
3.3.1.2 Helicobacter heilmannii Gastritis
This organism, formerly called Gastrospirillum hominis, is a pathogen of animals that occasionally infects humans (Oliva et al. 1993; Lavelle et al. 1994; Solnick et al. 1994; Akin et al. 1995; Debongnie et al. 1995; Yali et al. 1998). Symptoms include dysphagia and abdominal pain; antral or body erythema may be seen endoscopically. Nodularity of the mucosa as seen with H. pylori gastritis may be present (Mention et al. 1999). The gastritis is more focal, usually antral, chronically active, and less intense compared to that of H. pylori; the organisms are less associated with the mucin layer (Stolte et al. 1997; Singhal and Sepulveda 2005). They may be found in the lamina propria and even in parietal cells (Oliva et al. 1993). H. heilmannii are longer than H. pylori (5–9 μm versus 3 μm, respectively), spiraled as compared to bent, with bipolar as compared to the unipolar flagellae of H. pylori, and are detectable by H&E or with silver stains (Fig. 3.4). H. pylori and H. heilmannii infections may coexist (Svec et al. 2000; Ierardi et al. 2001). Infection with H. heilmannii may be treated like those of H. pylori or, if minimally symptomatic, untreated.
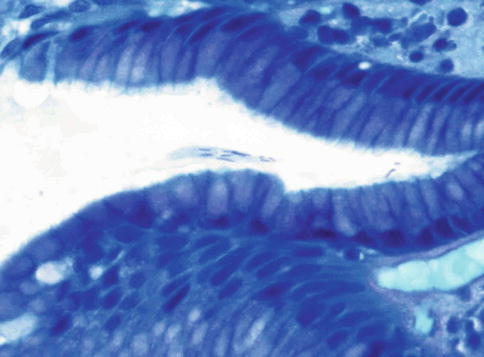

Fig. 3.4
Helicobacter heilmannii gastritis (Diff-Quik stain). Note the organisms are longer than H. pylori and straight rather than bent. The corkscrew twist is just discernable
3.3.1.3 Cytomegalovirus Gastritis
Cytomegalovirus (CMV), like herpes simplex virus, can cause an acute gastritis in the immunocompromised child and in infants. Erythema and erosions feature an acute inflammatory infiltrate, edema, cellular necrosis, and typical CMV inclusions. In children, CMV rarely causes a unique mimic of classic Ménétrier’s disease, with mucosal thickening due to prominent foveolar hyperplasia (Leonidas et al. 1973; Dempsey et al. 1992; Qualman and Hamoudi 1992; Takagi et al. 1992; Kovacs et al. 1993; Sferra et al. 1996) (Fig. 3.5). This unusual childhood disorder is discussed later in this chapter under Ménétrier’s disease.
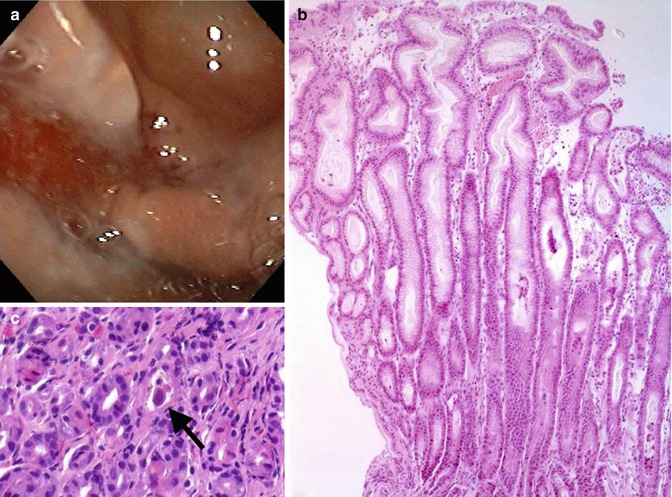

Fig. 3.5
Cytomegalovirus induced Ménétrier-like disease in a child. (a) Endoscopic view of thick gastric folds. (b) Marked foveolar hyperplasia. (c) CMV inclusion (arrow)
3.3.2 Other Infectious Agents
Larvae of the parasite Anisakis obtained from ingesting raw fish penetrate the gastric mucosa, causing abdominal pain and eosinophilic gastritis (Ikeda et al. 1989; del Pozo et al. 1999; Esteve et al. 2000). Giardia lamblia trophozoites can be identified in gastric biopsies where they may have no association with any particular type of pathological change (Oberhuber et al. 1997a). They seem more capable of surviving in the stomach when hypochlorhydria exists and, therefore, are sometimes found in adults with atrophic gastritis (Doglioni et al. 1992). Fungal infections such as mucormycoses do occur in premature infants and immunocompromised children (Michalak et al. 1980). Tuberculosis and syphilis can cause gastritis (Fyfe et al. 1993; Greenstein et al. 1994), and examples of streptococcal, staphylococcal, and Escherichia coli gastritis (phlegmonous) are known. Emphysematous gastritis due to gas-forming Clostridia sometimes complicates the immunosuppression of chemotherapy in pediatric oncology patients (Rowen et al. 1976). Gastritis may complicate varicella (Baker et al. 1973).
3.3.3 Noninfectious Immune Gastritides
3.3.3.1 Celiac Disease (and Lymphocytic Gastritis and Chronic Varioliform Gastritis)
The classic pathologic changes of celiac disease, that is, villus atrophy, crypt hyperplasia, lamina propria mononuclear cell inflammation, and T lymphocyte infiltration of the epithelium with evidence of cellular damage, predominate in the distal duodenum and jejunum. Sometimes abnormalities occur in ileum and rectum; the stomach, too, may be involved. In such a circumstance, the pathologist may suggest celiac disease when a lymphocytic gastritis pattern is present (Fig. 3.6).
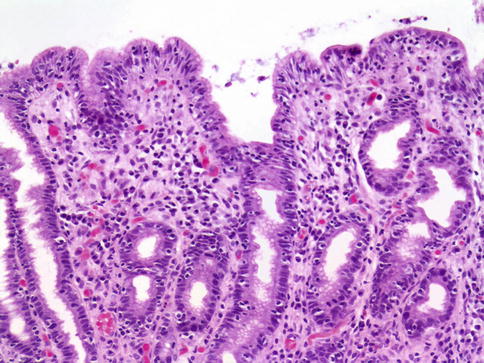

Fig. 3.6
Celiac disease. This antral biopsy sample shows chronic inflammation in lamina propria and lymphocytic permeation of surface epithelium
Lymphocytic gastritis, characterized by lymphocytosis of gastric epithelium (Farrell and Kelly 2002) and quantified as greater than 25 intraepithelial lymphocytes per 100 gastric columnar cells (Carmack et al. 2009), has an estimated prevalence in celiac disease between 10 and 45 % (Haot et al. 1988; Feeley et al. 1998; Diamanti et al. 1999; Jevon et al. 1999; Green and Jabri 2006). In these cases, the lymphocytosis is most commonly seen in the antrum (Haot et al. 1988; Oberhuber et al. 1999) and in patients with severe disease (Green and Jabri 2006). The association between lymphocytic gastritis and specific clinical or endoscopic findings in pediatric patients with celiac disease is unclear. In a review by Jevon et al. (1999) of 33 children with celiac disease, all had an endoscopically normal stomach and 29 had gastritis; 15 had both chronic inflammation of the superficial lamina propria and increased intraepithelial lymphocytes (IEL). Six had increased IEL alone. In this study, no relationship between histologic and clinical severity was found. However, in our review of 304 patients with celiac disease, we found that those patients with lymphocytic gastritis (39 out of 304) were statistically more likely to be diagnosed at an earlier age and present with more profound laboratory findings and duodenal mucosal damage compared to patients without gastric involvement (Bhatti et al. 2011). De Giacomo et al. (1994) found gastritis in 15 of 25 children with celiac disease, and of these, 9 had increased IEL (lymphocytic gastritis). Dyspeptic symptoms were more common in the latter group. Alsaigh et al. (1996) found no correlation of gastric pathologic severity to clinical symptoms or to serum antigliadin, antireticulin, or anti-endomysium levels. They noticed that the degree of gastritis correlated with that of the duodenum.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








