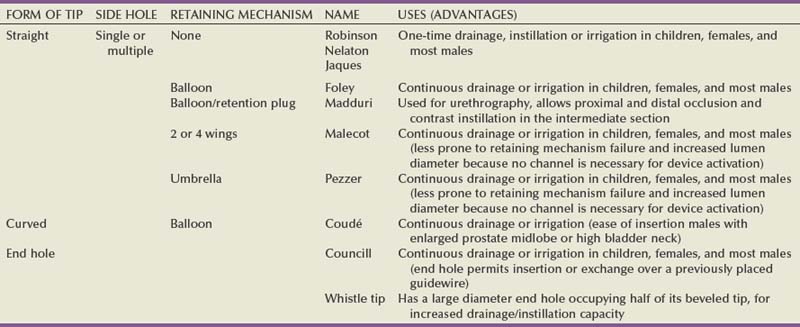
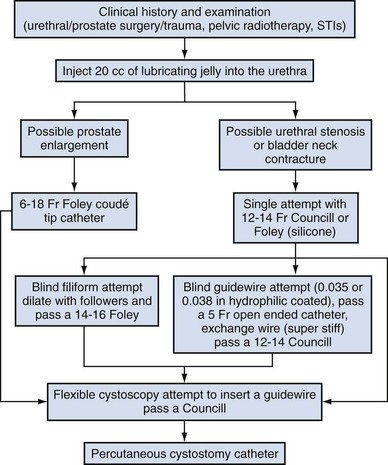
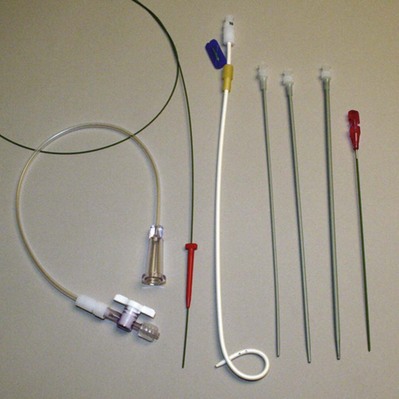
Carlos E. Méndez-Probst, MD, Hassan Razvi, MD, FRCSC, John D. Denstedt, MD, FRCSC, FACS Instruments to drain the urinary bladder are among the most ancient medical devices in the historical record. One of the earliest descriptions of a urinary catheter can be found in the Hippocratic text On Diseases (400 BC), in which bladder drainage was considered a basic skill in the armamentarium of Greek physicians (Moog et al, 2005a). The practice of bladder catheterization to permit urine drainage seems to have been widespread throughout the ancient world, with records of its application also found in India, Egypt, and the Byzantine and Roman empires. In Avicenna’s Canon of Medicine, mention is also made of urethral catheterization as a means to deliver intravesical therapy (Madineh, 2009). Surviving examples of early catheters include hollow tubes made of bronze, paper, animal hide, cloth soaked in wax, and lead (Moog et al, 2005b). In the 16th century, Ambroise Pare used catheters made of silver, brass, and copper. In the early 18th century, natural rubber was first used and the coudé tip was introduced by Mercier not long after. The self-retaining balloon catheter was introduced in the 20th century by Foley (Ellis, 2006), leading the way to permit development of catheters of synthetic materials with specialized coatings and drug-eluting properties. The size and type of urinary catheter used depends on the indication for catheter insertion, age of the patient, and type of fluid expected to be drained. Currently there is a wide variety of catheter types. Catheters can be classified on the basis of material, coating, number of channels (Fig. 7–1), and tip form (Fig. 7–2). Each of these characteristics is reviewed. Catheter size is measured in the Charrière or French scale, whereby one Fr or Ch is equal to 0.33 mm. This measurement indicates the total circumference of the catheter and not the lumen size. As a general rule, catheter size should be the smallest size that can accomplish the desired drainage (i.e., 12 to 14 Fr for clear urine and 20 to 24 for thick pus or blood-filled urine). In children, choosing a catheter of appropriate size is essential to avoid unnecessary urethral trauma (Table 7–1). The use of feeding tubes as urethral catheters should be discouraged because their stiffness and length can be a source of complications (ischemic ulcers, urethral strictures, and knotting in the bladder) (Smith, 2003; Robson et al, 2006). Table 7–1 Catheter Size Based on Age Modern urinary catheters are most frequently made of latex, rubber, silicone, and polyvinylchloride (PVC). Rubber and latex catheters are often chosen for short-term drainage. Silicone catheters are indicated when there is rubber/latex sensitivity or allergy and are particularly suited for patients requiring a longer period of indwelling time. Silicone is relatively inert, causing less tissue reaction, and is associated with less bacterial adherence than other catheter materials (Roberts et al, 1990). Evidence suggests that the use of silicone catheters is associated with a lower incidence of urinary tract infections compared with those made of latex (Crnich et al, 2007). Various coatings on urethral catheters have been applied in an attempt to reduce urethral trauma and infection risks. The use of hydrophilic coatings has been evaluated among patients on chronic self-intermittent catheterization, with some studies suggesting less discomfort, a decrease in symptomatic urinary tract infection (UTI) rates, and urethral strictures (Wyndaele J, 2002; Vapnek et al, 2003; De Ridder et al, 2005; Bjerklund et al, 2007). Thus far, the evidence is contradictory for the use of antiseptic-coated catheters to prevent the incidence of UTIs, with most of the studies to date having been restricted to small pilot studies (Johnson et al, 2006). The application of a viable bacterial coating onto catheter surfaces as a method of reducing catheter-associated urinary tract infection (CAUTI) by bacterial interference is a novel approach that has shown promise in a small pilot study involving the use of Escherichia coli–coated catheters. The rationale is based on natural competition by nonpathogenic bacteria overpowering any pathogenic bacteria that may enter the urinary tract (Trautner et al, 2007). Further study is necessary to confirm if this will be an effective strategy. Most catheters are designed with a blunt straight tip that is blind ending. Catheters with curved tips or with an end hole have specific utility in certain clinical scenarios (e.g., a coudé tip for patients with a high bladder neck or prominent median prostate lobe, a Councill catheter when catheterization over a guidewire is required). The various catheters and their typical applications are presented in (Table 7–2). The use of topical anesthetic gels before urethral catheterization is widely practiced; however; the evidence to support their use is conflicting. The agent used, temperature of the drug on administration, and indwell times before instrumentation are variable across the studies (McFarlane et al, 2001; Ho et al, 2003; Chung et al, 2007; Garbutt et al, 2008). There is some evidence that cooling to 4° C diminished the discomfort of lignocaine gel instillation, probably due to a cryo-analgesic effect (Thompson et al, 1999; Goel and Aron, 2003). Studies employing 2% lidocaine gel left indwelling at least 15 minutes have been reported to be associated with less pain (Siderias et al, 2004). If topical anesthesia is to be used, evidence suggests it requires a minimum of 10 minutes of exposure (depending on the agent), sufficient volume of the agent (20 to 30 mL), and slow instillation time (>3 to 10 seconds) (Schede and Thüroff, 2006; Tzortzis et al, 2009) to have the most effect. The systemic absorption of topical urethral lidocaine through intact mucosa is minimal, resulting in low measurable concentrations at doses of up to 550 mg (Ouellette et al, 1985). Rare toxicity has been reported, however, after traumatic procedures that disrupt the mucosal barrier, leading to high and rapid peak concentrations. Seizures, confusion, and disorientation, which usually precede cardiovascular toxicity (Sundaram, 1987; Priya et al, 2005), have been described. The male urethra follows a sigmoid curse, with a proximal curve at the junction of the membranous and bulbar urethra and another at the junction of the bulbar and penile urethra. The adult male urethra is approximately 18 to 20 cm in length, and its diameter is variable, from a mere slit to 6 mm during the passage of urine (Urinary system, 1995). The catheter should be attached to a sterile closed bag system as soon as urine is draining. The drainage bag should be placed below the level of the bladder to encourage one-way gravity flow with the tubing as straight as possible and avoiding kinks that might impair drainage. It has been shown that even the retention of 50 mL of urine in catheterized patients has been associated with an increase in UTIs in up to one third of the patients (Garcia et al, 2007). Catheterization in children is most commonly performed for drainage, performance of voiding cystourethrogram, or obtaining urine for culture. When attempting to obtain a urine sample for cultures, the use of a portable bladder ultrasound is recommended to ensure that an adequate amount of urine is present in the bladder, thus minimizing the risk of unproductive catheterization (Robson et al, 2006). Difficulty inserting a catheter into the bladder is most commonly due to prostatic growth, urethral stricture(s), bladder neck contracture, or false passage from previous urethral instrumentation. Rarely it is the result of phimosis or urethral calculi. Although these difficulties occur mostly in men, the techniques described herein may be applied to place a catheter regardless of gender (Fig. 7–3). If there is no clinical history of previous sexually transmitted infections (STIs), catheterization, trauma, urethral surgery, or radiotherapy in an adult male over 40 years of age, the most likely cause is prostatic enlargement. Using adequate urethral lubrication and a 16- or 18-Fr coudé tip silicone catheter is often successful in this scenario. If multiple previously unsuccessful attempts have been made and urethral trauma is suspected due to the appearance of a bloody urethral discharge, a false passage or a stricture is likely. A single atraumatic attempt can be made using a 12-Fr silicon/straight or coudé tip catheter. If this maneuver is unsuccessful, then depending on the availability of equipment and the level of experience of the clinician, several other options can be considered. The authors’ preference is to use a flexible cystoscope, allowing a direct visual approach that can be both diagnostic and therapeutic and minimizes the risk of further urethral injury. Under direct vision, the area where the false passage was created or the site of stricture formation is identified and an attempt is made to identify the true urethral lumen. Once identified, a 0.035 in Teflon-coated guidewire (e.g., Bentson type) is passed along the urethral lumen and into the bladder. Depending on the circumstances, urethral dilation may be required as in the situation of a tight urethral stricture or bladder neck contracture. Dilation can be accomplished over the guidewire using Amplatz dilators or purpose-built commercially available urethral dilators (Fig. 7–4A-C). Once the urethral lumen is deemed of adequate diameter to permit placement of the catheter size required, a Councill catheter, which can be advanced over the guidewire into the bladder, is selected. If flexible instruments are unavailable and a urethral stricture is suspected, then a blind technique. although less desirable, can be used. This technique involves using filiforms and followers (Fig. 7–5). If none of these techniques are successful, a suprapubic catheter should then be inserted. UTIs account for 40% of all nosocomial infections. The major risk factor is the use of urethral catheters, which are responsible for up to (80%) of UTIs in the hospital setting (Ha and Cho, 2006). Risk factors for CAUTIs include patients requiring more than 6 days of catheterization, female gender, active nonurinary infection sites, preexisting medical conditions, malnutrition, renal insufficiency, catheter insertion other than in the operating room, and having drainage tubing or a bag elevated above the level of the bladder (Maki and Tambyah, 2001). Avoidance of unnecessary catheterization, atraumatic technique at insertion, and the use of a closed collection circuit have been shown to decrease the incidence of CAUTI. The use of aseptic technique, although universally practiced and recommended by expert opinion, is not evidence based (Pratt et al, 2007). The use of antiseptic gels or irrigations of the bladder or collection bag, catheter clamping, or antireflux valves have not been shown to prevent CAUTIs (Phipps et al, 2006; Jahn et al, 2007; Tenke et al, 2008). Multiple trials to date have failed to show that hydrogel-coated catheters have a role in reducing the incidence of infections. The application of antibiotic coatings onto catheter surfaces and the concept of drug elution have shown some promise in preclinical investigations such as the application of nitrofurazone and minocycline/rifampin coatings, which have shown effectiveness in limited trials by reducing the incidence of asymptomatic bacteriuria (Lee et al, 2004; Stensballe et al, 2007). Evidence of the benefit of employing silver alloy coating to catheter surfaces has been conflicting (Davenport and Keeley, 2005; Srinivasan et al, 2006; Schumm et al, 2008). A recent Cochrane review reported a decrease in the rates of asymptomatic bacteriuria; however, whether this equates to a decrease in symptomatic infections is not clear (Jahn et al, 2007). The routine use of catheter coatings is currently not supported by the available literature. If the catheter has been indwelling for a long period of time, encrustation should be considered and imaging studies (plain film or ultrasound) will be confirmatory. In many instances the encrustation is easily dislodged with gentle traction on the catheter. For more significant encrustations, one can consider using a semirigid ureteroscope and the holmium:YAG laser to remove the stone fragments. If the catheter is quite rigidly fixed and in the setting of recent bladder or prostate surgery, semirigid ureteroscopy along the catheter and using the holmium:YAG to release the suture have also been described (Bagley et al, 1998; Nagarajan et al, 2005). Because the suture materials used in bladder and prostate surgery are often absorbable, waiting for suture dissolution is another option. If the balloon remains inflated, the problem is along the catheter’s inflation lumen or in the balloon itself. Although overinflation of the balloon has been described in an attempt to rupture the balloon, we do not recommend it because this maneuver may be painful to the patient and may cause bladder injury and fragmentation and retention of the balloon fragments (Gülmez et al, 1996). Similarly, the use of chemical instillations such as ether or toluene to induce balloon rupture should be discouraged because these agents can cause chemical cystitis (Patterson et al, 2006). Should none of these maneuvers be successful, the use of ultrasound-guided needle puncture can be conducted with a long spinal needle (22 gauge) using either a transrectal, transvaginal, or suprapubic surface probe (Daneshmand et al, 2002). In most instances, retained catheters can be removed without the need for open surgery. Other complications of urethral catheterization include hematuria, urethral and meatal strictures, urethral perforation, and allergic reactions including anaphylaxis (Thomas et al, 2009; Wyndaele, 2002). Especially at risk are patients with long-term indwelling catheters, in whom other complications may also include malignant neoplasms (2.3% to 10%), stone formation (46% to 53%), bladder neck and urethral erosions (Igawa et al, 2008). Suprapubic catheter (SC) insertion is most often selected for those patients in whom urethral access is not possible such as complete urethral stenosis, bladder neck contracture, and traumatic urethral disruption. Also, some patients who do not meet these criteria may be candidates for selective SC (Abrams et al, 2008), especially in the context of the need for a chronic indwelling catheter (i.e., neurogenic bladder, poor patient or cooperation/support for clean intermittent catheterization) because some evidence suggests that an SC may be associated with less discomfort than a urethral catheter (McPhail et al, 2006). The evidence that SC reduces the risk for symptomatic bacteriuria is relatively weak (Niël-Weise and van den Broek, 2005). Relative indications for SC placement also include patients who decline urethral catheterization for sexual or self-image purposes. Indications for short-term SC as a method of postoperative urinary diversion to allow bladder or urethral healing include following surgical procedures (e.g., open prostatic adenectomy, intestinal bladder substitution) or in the pediatric patients following reflux surgery, and during trans urethral prostate resection to theoretically prevent fluid absorption in high risk surgical patients (Sanchez Zalabardo et al, 2003). The distended bladder should be palpated or percussed to delineate its borders. Failure to palpate the bladder is a relative contraindication to blind percutaneous access techniques and should prompt the clinician to either wait until the bladder is more distended and palpable or use ultrasound imaging guidance for catheter placement (Aguilera et al, 2004). Because of the wide availability of portable ultrasound, ease of use, and added safety over the blind technique, we strongly advocate for its preferential use in percutaneous cystostomy placement to reduce possible complications. An alternative approach employs the Seldinger technique. An 18-gauge hollow needle is advanced under continuous aspiration with a syringe until urine flashback appears, confirming the bladder has been entered. The needle is then advanced 1 cm more, the syringe is detached from the needle, and a floppy-tip guidewire (0.035 or 0.038 in Bentson) is advanced and coiled in the bladder. The needle is withdrawn, a small incision is made on the anterior abdominal fascia, and the tract is coaxially dilated either in one step with a balloon-dilating catheter or with sequential graduated dilators (Fig. 7–6). A 16- to 18-Fr Councill catheter can then be advanced over the guidewire and into the bladder. (Courtesy of Cook Urological, Spencer, IN.) Contraindications to the use of a blind percutaneous approach are uncorrected coagulopathy, previous lower abdominal surgery, or pelvic radiation (Lawrentschuk et al, 2003). In these situations, an open technique with exposure of the anterior surface of the bladder should be used. The use of a retrograde simultaneous cystoscopy to manually displace the bladder dome and serve as a visual and palpatory reference in both the closed and open technique has been described in an effort to diminish the chance of bowel injury (Alagiri and Seidmon, 1998; Lawrentschuk et al, 2003; Sawant et al, 2009).
Lower Urinary Tract
For Urethral Catheterization
Historical Background
Catheter Selection
AGE IN YR
CATHETER SIZE (FR)
<5
5-8
5-10
8-10
10-14
10
>14
10-14
Material
Coating
Tip Shape
Anesthetic
Male Patients
Anatomic Considerations
Special Considerations in Children
Difficult Catheterization
Complications of Urethral Catheterization
Suprapubic Catheterization
Indications
Technique (Percutaneous)
Fundamentals of Instrumentation and Urinary Tract Drainage