Chapter 84B External-beam radiotherapy for liver tumors
Technical Innovations
Intensity-Modulated Radiotherapy
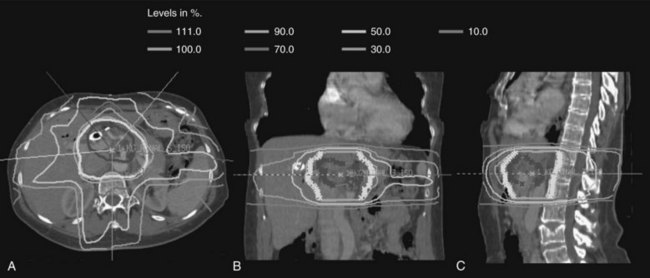
Over the last decade, more sophisticated imaging and treatment-planning techniques have allowed for more focal delivery of RT. Now routine is computed tomography (CT)-assisted three-dimensional conformal radiation therapy (3D-CRT), which uses diagnostic CT or magnetic resonance imaging (MRI) to define normal and target structures. IMRT is a further technical innovation, now frequently used in hepatobiliary radiation. As opposed to conventional radiation that uses large, fixed radiation portals and field borders based on correlations between the bony anatomy and the soft-tissue target, IMRT uses CT-based planning, and the target volumes and normal structures are delineated individually on axial CT imaging. With conventional RT, normal tissues adjacent to target structures cannot be spared and receive the prescribed radiotherapy dose, limiting the radiation dose that can be delivered safely. When planning treatment using IMRT, the radiation dose is prescribed to the target volume, and strict dose constraints are placed on normal tissues. The IMRT plan is then developed using a computer-optimized algorithm to deliver radiation of varying intensity to the target volume via multiple beams to meet the requirements for the target volume coverage and normal tissue constraints. The overall result is a highly conformal dose distribution customized to the shape of the tumor (Fig. 84B.1). This minimizes exposure of surrounding normal tissues and organs to high-dose radiation.
Image-Guided Radiotherapy and Motion Management
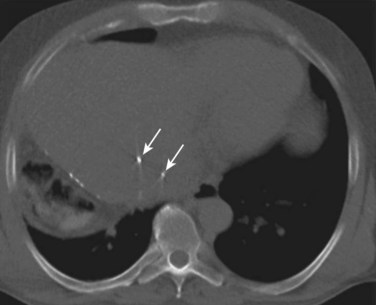
The development of 3D-CRT and IMRT, which allow for delivery of more conformal radiotherapy to the tumor, has made more accurate localization of the tumor at the time of treatment necessary to avoid missing the target. The need to improve targeting in radiation treatment has led to the development of image-guided radiation therapy to reduce the uncertainties of tumor positioning. On-board 3D CT imaging, known as cone-beam CT (CBCT), has been developed to allow real-time assessment of tumor positioning on the linear accelerator, while the patient is on the table prior to treatment delivery; however, CBCT scans may not show the target lesion in the liver because of limitations in resolution and contrast (Fig. 84B.2). Because liver tumors are not well visualized on standard megavoltage portal images or even on CBCT, surrogates for tumor location have been used to help verify tumor positioning prior to treatment. Fiducial markers (see Fig. 84B.2) have been introduced to help identify the tumor and allow for better visualization and improved setup for both conventionally fractionated treatments and, more importantly, for focal RT. Gold fiducial markers can be percutaneously inserted in or around the liver tumor, or postoperative clips can be used to help evaluate the postoperative bed on kilovoltage images. These fiducial markers are essential to allow for both tumor localization and assessment of tumor motion during treatment, and they are used in conjunction with many of the motion-management techniques described below.
Although 3D-CRT and IMRT offer the ability to tighten the margin around the target volume, the motion of the liver is complex because of organ deformation and rotation with respiration. Some series have found that the liver moves from 1 to 8 cm in the superior-inferior direction with respiration, and smaller shifts are noted in the anterior-posterior and left-right directions with breathing (Shirato et al, 2004). Because of this significant displacement over time, standard treatment techniques require large margins on the gross tumor volume to ensure a full dose to the tumor throughout the respiratory cycle.
Hepatocellular Carcinoma
Early on, researchers determined that low doses of large-field hepatic irradiation were ineffective in controlling gross hepatocellular carcinoma (HCC) and that higher doses of whole-liver irradiation resulted in high rates of radiation hepatitis. Stevens (1994) states that 75% of patients treated with 40 Gy or greater radiation to the whole liver will develop liver dysfunction, and an early study of adjuvant liver irradiation for HCC (Fugitt et al, 1973) demonstrated that 4 of 52 patients treated with more than 55 Gy had fatal hepatitis. These widely quoted early results have resulted in RT being placed on the list of potential palliative therapies for HCC.
With the development of 3D-CRT, high-dose, partial liver irradiation was also attempted for patients with unresectable liver tumors ( Dawson et al, 2000; Lawrence et al, 1993; Robertson et al, 1997). Investigators at the University of Michigan have reported a Phase II study of 3D-CRT and concurrent intraarterial floxuridine chemotherapy for unresectable hepatobiliary disease. They treated 128 patients with unresectable HCC (n = 35), intrahepatic cholangiocarcinoma (n = 46), or colorectal hepatic metastases (n = 47). Doses ranged from 40 to 90 Gy (median, 60.75 Gy) and were based on the amount of normal liver tissue, evaluated by dose-volume histograms, that could be effectively excluded from the fields to a maximum risk of radiation-induced liver disease (RILD) of 10% to 15%. At a median follow-up time of 16 months (26 months in patients who were alive) the median survival was 15.8 months, and the 3-year survival was 17%; in addition, 38 patients (30%) developed grade 3 or greater toxicity, and 5 patients developed RILD, one of whom died.
More recently, studies of external-beam radiotherapy (EBRT) using IMRT have been reported. A dose-escalation trial of conformal radiotherapy using 3D-CRT (n = 24) or IMRT (n = 16) was performed at Fudan University in Shanghai (Ren et al, 2010). The median tumor dose was 51 Gy (range, 40 to 62 Gy), and the median dose to normal liver was 17 Gy (range, 9 to 22 Gy). None of the patients experienced grade 3 or greater toxicity. The acute hepatic toxicities were mainly elevated liver enzymes; in-field progression-free rates at 1 and 2 years were 100% and 93%, respectively; overall survival at 1 and 2 years was 72% and 62%, respectively.
Investigators from the University of Virginia reported their experience treating 20 patients with HCC using IMRT to deliver 50 Gy in 20 fractions with concurrent capecitabine (McIntosh et al, 2009). The median tumor size was 9 cm, and 45% of patients had Child-Turcotte-Pugh (CTP) class B cirrhosis. The average volume of liver that received greater than 30 Gy was 27%. None of the patients experienced grade 3 or greater acute or late toxicity, and no cases of RILD were reported. The median survival for patients who had class A and B disease was 22.5 months and 8 months, respectively. These numbers exceed those quoted for radiation or regional chemotherapy alone and match most surgical series, they should therefore serve as the basis for future studies using dose escalation and radiation sensitizers.
Alternative fractionation schemes have also been introduced in the treatment of HCC. Both hyperfractionation (lower dose per fraction, twice-daily treatments) and hypofractionation (high dose per fraction in fewer fractions) have been applied to improve response rates in liver malignancies. The rationale for hyperfractionation is the rapid doubling time of HCC tumors (estimated at 41 days) and the shortened treatment times. Hyperfractionation was used in the University of Michigan series (Ben-Josef et al, 2005; Dawson et al, 2000; Lawrence et al, 1993; Robertson et al, 1997
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree