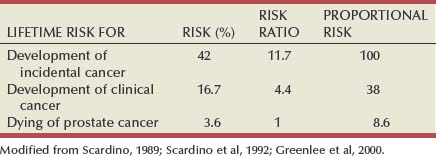
James A. Eastham, MD, Peter T. Scardino, MD, FACS The natural history of newly diagnosed prostate cancer is difficult to predict. Men with similar findings on digital rectal examination (DRE) and serum prostate-specific antigen (PSA) level (clinical stage), as well as histologic features on prostate biopsy (pathologic stage), can have markedly different outcomes. Whereas prostate cancer proves lethal in some patients, most men do die with, rather than of, their cancer. The ratio of approximately six newly diagnosed cases for every one death from prostate cancer each year—relatively constant during the past 30 years—is a result of the protracted natural history of the disease in most patients. Further highlighting the remarkable variation in behavior of this disease, autopsy studies have demonstrated that, on histologic examination, cancer can be found at autopsy in the prostate of approximately 42% of American men 50 years or older who die of causes other than prostate cancer; yet the lifetime risk that an American man will be diagnosed with prostate cancer is estimated to be about 17% and the risk of dying of the disease only 3.6% (Scardino et al, 1992; Sakr et al, 1993; Landis et al, 1999; Stanford et al, 1999) (Table 101–1). The challenge for physicians today is to identify the minority of men with aggressive localized prostate cancer that can be altered by definitive local therapy, while sparing the remainder of patients the morbidity of unnecessary treatment. Table 101–1 Lifetime Risk for Development of or Dying of Prostate Cancer for a 50-Year-Old Man in the United States Epidemiologic data demonstrate a high incidence of prostate cancer relative to historical norms and a profound migration toward earlier-stage disease at the time of diagnosis. Implementation of large-scale screening programs and more extensive biopsy strategies are largely responsible for this increase. A retrospective analysis of the control group in the Prostate Cancer Prevention Trial (a phase 3, randomized, double-blind, placebo-controlled study sponsored by the National Cancer Institute) illustrates the potential for indiscriminant biopsy strategies to result in overdiagnosis of prostate cancer (Thompson et al, 2004). Among 2950 men with normal findings on DRE and PSA levels less than 4 ng/mL, 15.2% were found by biopsy to have prostate cancer, including 6.2% of men with PSA levels of 0.5 ng/mL or less (Table 101–2). As the serum PSA thresholds for recommending prostate biopsy decrease and more extensive biopsy strategies result in stage migration toward earlier disease, appropriate management of the disease requires careful assessment of risk: How likely is a given man’s cancer to progress or metastasize during his remaining lifetime? What is the probability of success with treatment? What are the risks of side effects and complications with each treatment? Table 101–2 Prevalence of Prostate Cancer Found by Biopsy in the Placebo Arm of the Prostate Cancer Prevention Trial
| PSA LEVEL | NO. OF MEN (N = 2950) | MEN WITH PROSTATE CANCER (N = 449) NO. OF MEN (%) |
|---|---|---|
| ≤0.5 ng/mL | 486 | 32 (6.6) |
| 0.6-1.0 ng/mL | 791 | 80 (10.1) |
| 1.1-2.0 ng/mL | 998 | 170 (17.0) |
| 2.1-3.0 ng/mL | 482 | 115 (23.9) |
| 3.1-4.0 ng/mL | 193 | 52 (26.9) |
PSA, prostate-specific antigen.
From Klotz L. Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J Urol 2004;172:S48–50.
Watchful Waiting Versus Active Surveillance with Selective Delayed Definitive Therapy
Traditionally, watchful waiting has meant that no active treatment is begun until a patient develops evidence of symptomatic disease progression, at which time androgen deprivation therapy is initiated. The goal of this approach is to limit morbidity from either the disease or the therapy, not to administer potentially curative treatment (Table 101–3). The assumption is that definitive local therapy provides little or no benefit to the majority of men diagnosed with prostate cancer.
Table 101–3 Contrasts between Active Surveillance and Watchful Waiting
| ACTIVE SURVEILLANCE | WATCHFUL WAITING | |
|---|---|---|
| Aim | To individualize treatment | To avoid treatment |
| Patient characteristics | Fit for radical treatment; Age 50-80 yr | Age >70 yr or life expectancy <15 yr |
| Tumor characteristics | T1-T2, GS ≤7, initial PSA <15 ng/mL | Any T stage, GS ≤7, any PSA |
| Monitoring | Frequent PSA testing | PSA testing unimportant |
| Repeated biopsies | No repeated biopsies | |
| Indications for treatment | Short PSADT | |
| Higher grade/more extensive cancer on repeat biopsy | Symptomatic progression | |
| Treatment timing | Early | Delayed |
| Treatment intent | Radical | Palliative |
GS, Gleason sum; PSA, prostate-specific antigen; PSADT, PSA doubling time.
From Parker C. Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol 2004;5:101–6.
An alternative and more widely practiced strategy is to delay curative local therapy until the natural history and threat posed by the cancer can be more accurately characterized. Active surveillance (AS) with selective delayed definitive therapy attempts to distinguish clinically insignificant cancers from life-threatening cancers while they are still localized and potentially curable. This approach weighs the threat posed by the cancer within the patient’s remaining lifespan and the risks of definitive, local therapy, continually reassessing the risk-benefit ratio at each evaluation. The assumption is that the risk posed by a given cancer can be assessed with some degree of certainty. The goal is to avoid overtreating the majority of patients while administering curative therapy when needed in selected cases (Table 101–3). The risks are the delayed therapy may be less effective or would need to be more intensive, with greater morbidity, to be as effective as immediate treatment.
Watchful Waiting
Many clinicians recommend watchful waiting for the treatment of prostate cancer in elderly men with competing comorbidities. Several investigators have attempted to document the long-term risk of metastatic disease and death for men whose clinically localized prostate cancer was treated conservatively (Adolfsson et al, 1994; Chodak et al, 1994; Albertsen et al, 1995, 1998, 2005; Johansson et al, 1997, 2004; Cuzick et al, 2006). The impact of this slow-growing cancer depends on the life expectancy (age and comorbidities) of the patient and the clinical features (Gleason score, PSA) of the cancer (Table 101–4). Those patients expected to live less than 5 years may develop local symptoms, but few patients will develop metastases or die of the disease.
Table 101–4 Life Expectancy of Men (All Races) in the United States by Age in 5-Year Increments
| AGE | LIFE EXPECTANCY (YR), 1999-2001 |
|---|---|
| 50 | 27.8 |
| 55 | 23.6 |
| 60 | 19.7 |
| 65 | 16.1 |
| 70 | 12.8 |
| 75 | 9.9 |
| 80 | 7.4 |
| 85 | 5.5 |
Modified from Arias E, Curtin LR, Wei R, Anderson RN. United States decennial life tables for 1999-2001, United States life tables. National vital statistics reports; vol 57 no 1. Hyattsville (MD): National Center for Health Statistics; 2008.
The risk of metastases or death from clinical stage T1 to T2 cancer managed conservatively was estimated in a meta-analysis of six watchful waiting series (Chodak et al, 1994). The risk of metastasis at 10 years was 19% for well-differentiated cancers, 42% for moderately differentiated cancers, and 74% for poorly differentiated cancers. Furthermore, if the primary tumor was not controlled, the risk for development of metastases persisted, even accelerated, after 10 years. These findings were confirmed by Johansson and colleagues (2004), who reported their long-term results among men with early-stage prostate cancer (T1-T2, NX, M0) or watchful waiting. They observed a consecutive sample of 223 men for a mean period of 21 years. Most cancers had an indolent course during the first 10 to 15 years. However, further follow-up from 15 to 20 years revealed a substantial decrease in cumulative progression-free survival (from 45% at 15 years to 36% at 20 years), survival without metastases (from 77% to 51%), and prostate cancer-specific survival (from 79% to 54%) (Fig. 101–1). The authors concluded that although most prostate cancers diagnosed at an early clinical stage have an indolent course, local tumor progression and aggressive metastatic disease may develop in the long term and early local treatment should be considered for men with a life expectancy exceeding 15 years.
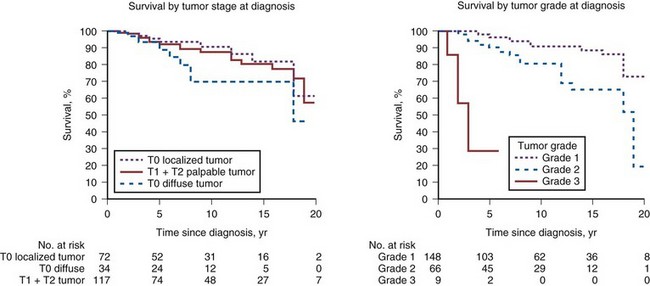
(From Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA 2004;291:2713–9.)
The impact on mortality was further documented in a large, population-based study (Albertsen et al, 1998, 2005). The probability of death from prostate cancer within 20 years of diagnosis depended on the Gleason sum of the cancer and the age of the patient at diagnosis. Patients with a well-differentiated cancer (Gleason sum 2 to 4) had a low probability of death from cancer within 20 years (6 deaths per 1000 person-years; 95% confidence interval [CI], 2-11); higher-grade cancers took a substantial toll even among older men (Gleason score of 8 to 10, 121 deaths per 1000 person years; 95% CI, 90-156). In contrast to Johannsen, the Albertson cohort did not experience acceleration in the rate of metastases or death from cancer between 15 and 20 years.
Kattan and colleagues (2008) reviewed United Kingdom cancer registry data to determine predictors of long-term outcomes for men with clinically localized prostate cancer managed without curative intent in the PSA era. The study included men who at the time of diagnosis were aged 30 to 75 years (inclusive), had a baseline serum PSA level of less than 100 ng/mL, had no evidence of metastatic disease, were not treated with curative intent, and were alive for at least 6 months after diagnosis of their disease. Diagnosis was by transurethral resection of the prostate in 1358 (56%) and needle biopsy in 1088 (44%). Most men were managed without initial treatment, while 30% were treated with hormone therapy within 6 months of diagnosis. In a competing risk analysis, 30% of the patients died of prostate cancer and 30% of other causes within 12 years. This was the first long-term watchful waiting study to include PSA at diagnosis and Gleason grade (reviewed centrally). Figure 101–2 shows the 10-year probability of death from prostate cancer and death from any cause by PSA and Gleason score at diagnosis (Cuzick et al, 2006). However, among men who did not die of another cause in this cohort, the probability of metastases, death from prostate cancer, or additional therapy within 12 years was 90%. These results suggest that localized prostate cancer detected clinically, in an unscreened population, results in a high rate of progression or secondary treatment within 12 years when managed with watchful waiting. In these watchful waiting cohorts, age, Gleason grade, and PSA were able to identify men at increased risk for the development of metastatic disease and death from prostate cancer. Nevertheless, not all men with “adverse” features do poorly, nor do all men with favorable features do well. Additional clinical and pathologic parameters are necessary to predict outcomes more accurately and to place expectant management of some patients on a sound basis.
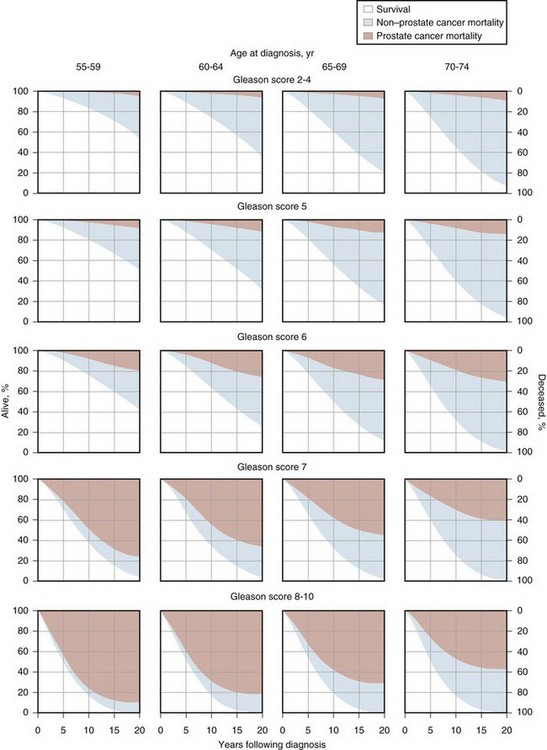
(From Cuzick J, Fisher G, Kattan MW, et al. Long-term outcome among men with conservatively treated localized prostate cancer. Br J Cancer 2006;95(9):1186–94.)
Watchful Waiting Versus Treatment
Perhaps the most compelling evidence that selected patients with prostate cancer benefit from active treatment compared with watchful waiting comes from the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4), which randomized 695 men with clinically localized prostate cancer to either radical prostatectomy or watchful waiting, with systemic treatment deferred until the development of symptomatic progression (Bill-Axelson et al, 2005, 2008). During a median of 10.8 years of follow-up, 47 of the 347 men (13.5%) who were randomly assigned to surgery died of prostate cancer, as did 68 of the 348 men (19.5%) assigned to watchful waiting (Fig. 101–3). At 12 years, 12.5% of the surgery group and 17.9% of the watchful waiting group had died of prostate cancer (difference = 5.4%, 95% CI, 0.2 to 11.1), for a relative risk of 0.65 (95% CI = 0.45 to 0.94; P = .03), and 19.3% in the surgery group and 26% in the watchful waiting group had distant metastases (difference = 6.7%, 95% CI = 0.2 to 13.2), for a relative risk of 0.65 (95% CI = 0.47 to 0.88; P = .006). This elegant study, which was carried out in a population not screened for prostate cancer with PSA, documents the overall benefit of radical prostatectomy for patients whose cancer is detected clinically. Because the estimated lead time for PSA screen–detected cancers is 6 to 8 years compared with clinically detected prostate cancers, the relevance of the Scandinavian study to the cancers detected by screening is uncertain.
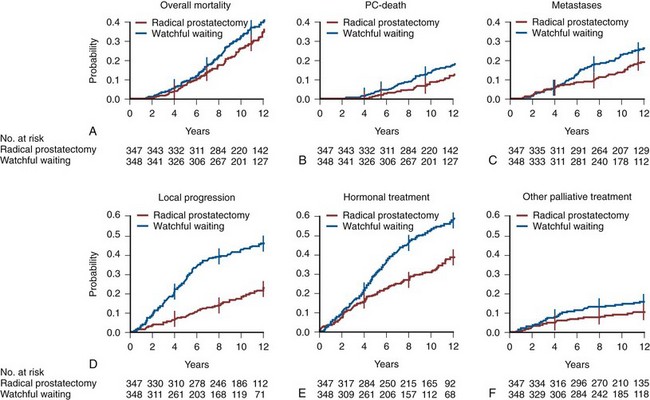
(From Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst 2008;100(16):1144–54.)
Although the development of metastatic disease and death from prostate cancer are important adverse outcomes, local cancer progression and the need for treatment also have significant impact on quality of life (Fig. 101–3). For example, the Scandinavian Prostate Cancer Group reported that fewer men in the radical prostatectomy group than in the watchful waiting group developed local recurrence or disease progression (Steineck et al, 2002; Bill-Axelson et al, 2008). At 12 years, the cumulative incidence of local recurrence and/or progression was 21.7% in the surgery group and 45.6% in the watchful waiting group (difference = 23.9, 95% CI = 16.8 to 30.9). A total of 132 men in the radical prostatectomy group versus 205 in the watchful waiting group started hormonal therapy. The difference in cumulative incidence of hormonal therapy at 12 years was 19.3% (95% CI = 11.7 to 26.9), with a lower probability of starting hormonal therapy in the radical prostatectomy group. All other palliative treatments (including radiation, treatment with cytotoxic drugs, and laminectomy) were also less common in the radical prostatectomy group. The difference in the cumulative incidence of palliative treatment at 12 years was 5.2% (95% CI = 0.1 to 10.3). Erectile dysfunction and incontinence were higher in men treated with radical prostatectomy, whereas men on watchful waiting experienced significantly more obstructive voiding complaints and bowel problems, resulting in similar levels of treatment-related distress (Table 101–5).
Active Surveillance
Widespread use of PSA screening, combined with low thresholds for recommending prostate biopsy and more aggressive biopsy techniques (12 core, saturation), have resulted in a profound shift toward detection of prostate cancer earlier (Draisma et al, 2009). Furthermore, large randomized trials of PSA screening have shown a limited impact on cancer specific mortality (Andriole et al, 2009; Schroder et al, 2009). These studies suggest that a proportion, estimated in one study as 23% to 42% (Draisma et al, 2009), of prostate cancers are being detected by PSA screening that would never have been detected in the subjects’ lifetime. These cancers are considered by some to be “overdetected,” and this concept has led to wider acceptance of AS for low-risk prostate cancers.
Identifying Men with “Low-Risk” Prostate Cancer
Systematic Biopsy Results
In an attempt to identify patients who have low-risk cancer, Epstein and colleagues (1994) examined preoperative clinical and pathologic features in 157 men with clinical stage T1c prostate cancer (who later underwent radical prostatectomy) to find features that could predict “insignificant” cancer (pathologically organ-confined, < 0.2 mL, Gleason sum ≤ 6). Their model for predicting an insignificant cancer included no Gleason grade 4 or 5 in the biopsy specimen and either:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









