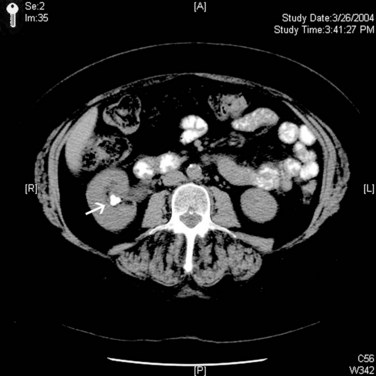
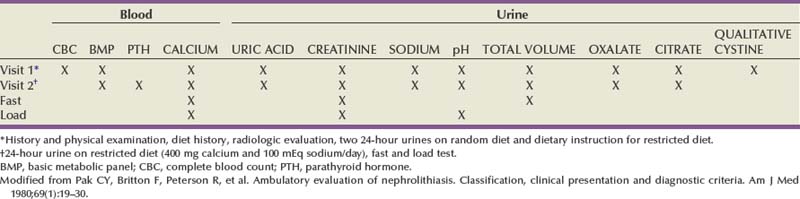
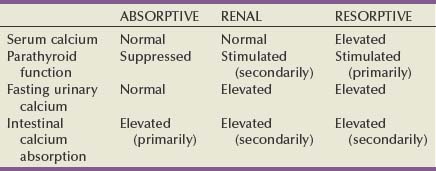
Michael N. Ferrandino, MD, Paul K. Pietrow, MD, Glenn M. Preminger, MD Symptomatic urinary calculi are undoubtedly associated with significant patient discomfort. Despite the ability of many stones to pass spontaneously, the surgical treatments for calculi may themselves be morbid. Patients may suffer financial pain due to the expense of emergency department visits, office visits, surgical procedures, or time lost from work, in addition to the physical sequelae. Logically, most patients are interested in learning how to prevent a recurrence of such an episode. Through even a rudimentary understanding of the physiologic causes of urinary calculus formation, physicians may offer a straightforward approach to elucidating the metabolic basis of nephrolithiasis for any given patient. This evaluation should be simple to perform, it must be economically viable, and it should provide information that can be applied toward a selective, rational therapy of stone disease (Pak et al, 1980a). Any evaluation should be able to identify associated metabolic disorders responsible for recurrent stone disease. These metabolic problems include distal renal tubular acidosis, primary hyperparathyroidism, enteric hyperoxaluria, cystinuria, and gouty diathesis. In many of these relatively uncommon conditions, it is generally agreed that selective medical therapy is indicated not only to prevent further stone formation but also to correct underlying physiologic disturbances that may lead to nonrenal complications (Pak et al, 2002a, 2003a). Debate continues regarding which patients require an extensive metabolic evaluation. First-time “stone formers” have often been estimated to have a 50% risk of recurrence within the subsequent 10 years (Uribarri et al, 1989). In two separate studies, Ljunghall and colleagues attempted to measure the incidence of a stone recurrence in a Northern European population. A retrospective review estimated the chance of recurrence as nearly 50% at 5 years, whereas a prospective evaluation noted a lower overall rate of 53% within 8 years (Ljunghall and Danielson, 1984; Ljunghall, 1987). Males had both a higher incidence of calculi overall, as well as a higher recurrence rate. Patients had a higher risk of repeat stones in the years immediately following their first episode. It is not completely apparent whether these were preexisting stones that passed later or whether they represent the formation of new calculi. In fact, recent evidence suggests that the incidence of stone disease may be escalating, along with an increasingly higher percentage of female stone formers (Scales et al, 2007). In fact, the largest epidemiologic study to date suggests the once 3 : 1 male-to-female ratio of stone formers has now decreased to 1.3 : 1, not due to a decrease in male stone formation but a significant increase in female stone formation (Pearle et al, 2005). This finding is thought to be due to changes in diet and lifestyle. Considering that dietary and fluid manipulation alone can reduce rates of stone recurrence, some suggest that first-time stone formers should be provided empiric fluid and dietary recommendations until they suffer a recurrence (Borghi et al, 1996). Indeed, studies of “single stone formers” placed on a conservative program of high fluid intake alone or combined with avoidance of dietary excess revealed a low incidence of recurrent stone disease (Hosking et al, 1983). Calling this finding the “stone clinic effect,” Hosking and colleagues noted metabolic inactivity in nearly 60% of all patients followed for more than 5 years. In comparison, Pak has found that single stone formers have an equally high incidence of metabolic abnormalities as recurrent stone formers (Pak, 1982). Furthermore, these derangements are just as severe, leading the authors to conclude that single stone formers should undergo the same evaluation as recurrent stone formers. Similar findings were reported in a series of 182 patients, where half of the patients had hypercalciuria or hyperuricosuria, whereas roughly 20% had a systemic disorder that predisposed the patients to the formation of calculi (Strauss et al, 1982a). The remainder, 29.1%, had no metabolic disorder. Patients with single stones tended to be older when they passed their stones and required a greater rate of intervention to treat the calculus. The recurrence for both groups of patients was similar (≈10% at 3 years). Because the authors did not note substantial differences between solitary and recurrent stone disease, they recommended that first-time stone formers should be evaluated similar to patients with recurrent stone disease. This approach has been partially refuted by Yagisawa and colleagues (1998), who noted that men with recurrent calculi had a higher rate of metabolic derangements than first-time stone formers. Although women had a trend toward the same pattern, this only achieved statistical significance with regards to decreased levels of urinary citrate (hypocitraturia) (Yagisawa et al, 1998). A more complete discussion of the economic aspects surrounding the decision to perform a metabolic evaluation is found later in this chapter. Importantly, the formation of a first stone may be the harbinger of a more severe underlying systemic disorder such as renal tubular acidosis, bone disease, or hypercalcemia due to hyperparathyroidism. In such patients, metabolic evaluation is justified solely to make the correct diagnosis in order to prevent extrarenal complications. With the development of reliable parathyroid hormone assays, it is unacceptable to wait for bone loss before embarking on curative therapy. Although the clinical significance of normocalcemic hyperparathyroidism has been questioned and is frequently simply observed, current practice favors the treatment of those patients with at least 1 mg/dL above the upper limit of normal, marked hypercalciuria (urinary calcium excretion > 400 mg per day), reduced bone density, and an age of younger than 50 years (Bilezikian and Silverberg, 2004). The decision to thoroughly investigate a first-time stone former should ideally be shared by the physician and the patient. Although some first-time stone formers will readily accept and follow conservative therapy, others may elect to undergo a thorough evaluation. It is quite reasonable to determine the extent of evaluation according to the estimation of potential/risk for recurrent stone formation (Smith, 1984). Patients at higher risk for repeat episodes are those with a family history of stones, those with intestinal disease (particularly when causing chronic diarrheal states), pathologic skeletal fractures, osteoporosis, urinary tract infection or gout. In these patients, an extensive evaluation is recommended. Any patients with stones composed of cystine, uric acid, or struvite should undergo a complete metabolic workup. All children should be required to undergo a complete investigation because they have been found to have a significant risk of underlying metabolic disturbances (Polito et al, 2000; Tekin et al, 2001; Pietrow et al, 2002; Coward et al, 2003; Bartosh, 2004). Additionally, these young patients have more at stake because early, repeated episodes of urinary obstruction, urinary tract infection, and repeated radiographic imaging all have associated morbidities. African-Americans have previously been observed to have a significantly lower incidence of nephrolithiasis than their Caucasian counterparts. Indeed, in a study by Sarmina and colleagues (1987), white patients had urinary calculi three to four times as often as black subjects. Notably, the male-to-female prevalence is not as drastic as it is for white subjects (1 : 1.55 compared with 2.3 : 1, respectively). This finding is taken one step further in a study by Michaels and colleagues (1994), in which the women made up roughly 60% of the African-American patients, thereby reversing the expected gender ratio. Sarmina found a higher incidence of infection calculi in the African-American population, whereas these stone types were excluded from analysis in the study by Michaels. Additional support for ethnic diversity is provided by Mente and colleagues (2007). Compared with Europeans, East Asian and African patients had a decreased relative risk of calcium nephrolithiasis, whereas Arabic, West Indian, West Asian, and Latin American patients had an increased relative risk. The authors found differing urinary profiles for a variety of the ethnicities reported when compared with Europeans. However, despite the decreased risk of calcium stone formation, patients of African ancestry demonstrated no significant differences in urinary metabolic derangements. Following the assumption that a lower incidence of calculi might imply a significant risk of a metabolic or anatomic abnormality in those patients that still manage to make calculi, it seems reasonable to advocate for the performance of a metabolic evaluation for all patients of African-American descent. This suggestion is supported by recent studies that assessed the underlying metabolic abnormalities of non-Caucasian stone formers. African-Americans, Asians, and Hispanics appear to have a surprisingly similar incidence of underlying metabolic disturbances when compared with Caucasian stone formers. These results suggest that dietary and environmental factors may be as important as ethnicity in the etiology of stone disease (Beukes et al, 1987; Maloney et al, 2005). Regardless of whether a particular, individual patient requires a full metabolic evaluation, it is prudent to perform at least a screening evaluation combined with a thorough history and physical examination to assess for underlying systemic syndromes that may cause recurrent calculi and extrarenal complications. This assessment should also screen for those patients at an increased risk of stone recurrence as outlined in the previous paragraphs (Table 46–1). Table 46–1 Indications for a Metabolic Stone Evaluation Key Points: Selection of Patients for Metabolic Evaluation In single stone formers without increased risk for recurrence, the following abbreviated protocol may be applied (Table 46–2). A thorough medical history should be obtained for any underlying conditions that may have contributed to the stone disease. Due to the association between bowel disease and calcium oxalate nephrolithiasis (enteric hyperoxaluria), a careful history of bowel habits and bowel disease should be sought (Smith et al, 1972; Bohles et al, 1988; Lindsjö et al, 1989; McConnell et al, 2002; Worcester, 2002; Parks et al, 2003b). This includes questions regarding chronic diarrhea that could be caused by inflammatory bowel disease (Crohn, ulcerative colitis) or irritable bowel syndrome. A history of gout should be sought because this finding may predispose the patient to hyperuricosuria or gouty diathesis with either uric acid calculi or calcium oxalate stone formation (Grover and Ryall, 1994; Khatchadourian et al, 1995; Kramer and Curhan, 2002). As described by Pak and colleagues (2003c), patients with a history of diabetes mellitus may be at an increased risk of developing a gouty diathesis, with altered ammonium management, acidic urine, and a predisposition for a mixture of calcium oxalate and/or uric acid stones. Table 46–2 Abbreviated Evaluation of Single Stone Formers In addition, information should be obtained concerning the patient’s dietary habits including fluid consumption and excessive intake of certain foods, as well as a list of all medications taken. A social history may provide obvious clues regarding a patient’s hydration status. Do they have access to fluids on a regular basis or are they sequestered along an assembly line or in the operating suite? Does the patient perform daily tasks that would increase their insensible losses of fluids (manual labor, prolonged outdoor exposure, excessive exercise)? Some have suggested that a sedentary lifestyle may actually increase the risk of stone formation over those who perform manual labor. Indeed, patients on prolonged bedrest demonstrate alterations in urinary chemistry such that urinary calcium and phosphorous excretion increase significantly, leading to significant increases in urinary saturation of calcium phosphate, calcium oxalate, and monosodium urate (Hwang et al, 1988). A family history may reveal a genetic predisposition to urinary calculi if there is a history of close relatives affected by nephrolithiasis. Age of onset of the patient or of affected relatives may give clues regarding genetic disorders such as autosomal recessive cystinuria. The urine sediment should be examined for crystalluria because particular crystal types may give a clue as to the composition of stones the patient is forming. Tetrahedral “envelopes” are seen in calcium oxalate lithiasis (Fig. 46–1), whereas rectangular, “coffin-lid” crystals are often seen in patients with struvite calculi (see Fig. 46–1). Hexagonal crystals confirm cystinuria (see Fig. 46–1), whereas uric acid crystals may be seen as amorphous fibers or irregular plates. The microscopic appearances of common calculi are summarized in Table 46–3. (Courtesy of Dr. S.R. Khan, University of Florida, Gainesville.) Table 46–3 Microscopic Appearance of Common Urinary Calculi Urine cultures are performed if there is a suspicion of infection-related calculi or if there are signs or symptoms of a urinary tract infection. A culture that is positive for urea-splitting organisms such as Proteus, Pseudomonas, and Klebsiella would help explain the formation of a struvite calculus. A positive culture will also warrant therapy with appropriate antibiotics before the initiation of any surgical procedure to remove the stone. The surgical management of a calculus during an active infection will place the patient in great risk of bacteremia or sepsis. Unfortunately, many infected calculi will harbor bacteria even after treatment with broad-spectrum antibiotics. Rocha and Santos demonstrated that bacteria can still be cultured from the interior of a calculus despite being soaked in iodine and alcohol for 6 hours (Rocha and Santos, 1969). Furthermore, McAleer and colleagues (2003) have shown that infection calculi contain large quantities of endotoxin after disintegration. In a comparison of infected versus noninfected calculi, infected stones contained 36 times more endotoxin. Half of the infected calculi grew bacterial cultures that were different from the preoperative urine specimens. The same investigators have described how endotoxin can cause a vascular collapse because it induces physiologic changes consistent with septic shock (McAleer et al, 2002). Abdominal radiographs should be obtained to document the existence of any residual stones within the urinary tract. The radiopacity of any existing stones may suggest the type of stones that are present. Although magnesium ammonium phosphate and cystine stones are often radiopaque, they are not as dense as calcium oxalate or calcium phosphate stones. A plain abdominal film is also useful in identifying nephrocalcinosis (suggestive of renal tubular acidosis) and staghorn calculi (likely due to infection lithiasis). An intravenous pyelogram may be obtained to confirm the presence of radiolucent stones and also identify any anatomic abnormalities that may predispose the patient to stone formation. It is important to realize that the radiographic evaluation of a patient during a metabolic workup of the stone disease will differ from an approach taken during an episode of acute renal colic. In these instances, patients are frequently examined with multislice, noncontrasted computed tomography (CT), which is able to quickly image the entire collecting system in a rapid sequence (Fig. 46–2) (Smith et al, 1995; Sommer et al, 1995; Katz et al, 1996; Fielding et al, 1997). Finally, available stones should be analyzed to determine their crystalline composition. The presence of uric acid or cystine would suggest the presence of gouty diathesis or cystinuria, respectively. The finding of struvite, carbonate apatite, and magnesium ammonium phosphate would suggest infection lithiasis. A predominance of a hydroxyapatite component suggests the presence of renal tubular acidosis or primary hyperparathyroidism and warrants an assessment of basic electrolytes. Stones composed of pure calcium oxalate or mixed calcium oxalate and hydroxyapatite are less useful diagnostically because they may occur in several entities including absorptive and renal hypercalciuria, hyperuricosuric calcium nephrolithiasis, enteric hyperoxaluria, hypocitraturic calcium nephrolithiasis, and low urine volume (Kourambas et al, 2001; Pak et al, 2004). Key Points: Abbreviated Protocol for Low-Risk Single Stone Formers Pak initially described an extensive outpatient (ambulatory) evaluation in 1980 and subsequently made minor revisions to help simplify the process (Pak et al, 1980a; Levy et al, 1995). The basic strategy involves two outpatient visits, and most of the required laboratory analyses can be performed in a routine clinical laboratory with only a few of the specialized techniques being performed in a more sophisticated laboratory. The entire schedule of visits and tests is outlined in Table 46–4. A third 24-hour sample may be collected after 1 week with the patient on a calcium-, sodium-, and oxalate-restricted diet. This dietary restriction is imposed to standardize the diagnostic tests, to better assess the etiology of hypercalciuria (i.e., AH I vs. AH II) and to prepare for the “fast and calcium load” test, which is performed on the second visit. Blood samples are obtained on both visits as outlined in Table 46–4. Due to the similar treatment of patients with absorptive hypercalciuria and renal leak, the performance of fast and calcium load testing is no longer performed by most clinicians. With little therapeutic distinction, there is not much of an incentive to discriminate between either type of hypercalciuria. However, differentiation between absorptive and renal hypercalciuria is essential if one plans to place a patient with absorptive hypercalciuria on a calcium-binding resin (see “Selective Medical Therapy of Nephrolithiasis” later in this chapter). A description of the “fast and calcium load” study is included here primarily for completeness and historical purposes. A “fast and calcium load” study is performed on the morning of the second visit (Pak et al, 1975). The purpose of this exercise is to help delineate between various causes of hypercalciuria. As explained in Chapter 45 in greater detail, some patients are too efficient at absorbing calcium from the alimentary canal (absorptive hypercalciuria, types 1 and 2), whereas others suffer from a constant loss of calcium from the renal tubules (renal calcium leak). A third subset of patients has an overabundance of circulating parathyroid hormone, usually from a single parathyroid adenoma, and has a constant loss of calcium and phosphate (resorptive hypercalciuria or primary hyperthyroidism). Key Points: Extensive Diagnostic Evaluation Several authors have suggested a more simplified approach that uses the same standard principles and procedures as the full outpatient evaluation. These simplified protocols do not include the calcium fast and loading tests and may not require adherence to a restricted diet, permitting them to be performed in a single office visit. Rivers and colleagues (2000) have recommended the collection of two separate 24-hour urine specimens. One is collected while on a restricted diet, whereas the other allows a random (typical) diet (Rivers et al, 2000). Such manipulation is well tolerated by the patient and may allow for identification of the various types of hypercalciuria with reasonable certainty. Pak has also recognized the cumbersome nature of an extensive evaluation and has made similar recommendations (Pak, 1997). On the basis of the findings of a single 24-hour urine collection, patients are evaluated and treated without all steps of fasting and loading calcium challenges. Patients are separated into complicated and uncomplicated calcium stone disease on the basis of the presence or absence of normocalcemia, normouricemia, and calcium stones and the absence of urinary tract infection, bowel disease, or marked hyperoxaluria. Comprising the majority of all patients, uncomplicated calcium stone disease is further separated into a hypercalciuric group and a normocalciuric group. Medical therapy is then based on this distinction. Lifshitz and colleagues (1999) have also advocated for a less complex approach. All patients undergo a basic metabolic screening, searching for systemic disorders that could pose a long-term health risk. They suggest that all patients should be advised about conservative nonspecific preventive measures. High-risk stone patients should have a more extensive metabolic evaluation based on two 24-hour urine samples. The cornerstone of these simplified protocols has been the development of a urine preservation method that allows collection of urine without refrigeration. The patient is then able to submit an aliquot to a central laboratory for the analysis of various stone-forming substances (Nicar et al, 1987). The urinary constituents most commonly assayed include calcium, oxalate, citrate, total volume, sodium, magnesium, potassium, pH, uric acid, and sulfate. Although most of these parameters are self-evident, sulfate is added to the list to assess the volume of protein loading from animal meat. From such determinations, the urinary saturation with respect to stone-forming salts can be calculated. At present, there are multiple private companies that offer laboratory services focused on simplified, accurate 24-hour urine assessment for stone-forming risk factors. A list of some of the more commonly used commercial laboratories is included in Table 46–5. The laboratories provide collection containers with chemical preservatives (obviating iced storage and transport) and extrapolate 24-hour cumulative data from the submission of a small aliquot of the entire collection. After the values of all urinary constituents and saturations have been determined, the physician receives a computerized printout that provides a numeric display of the test results (Fig. 46–3). A graphic display of this information may also be generated, highlighting the increased or reduced risk for each environmental, metabolic, or physicochemical factor (Fig. 46–4). These results should aid the physician in formulating a metabolic/physiologic diagnosis. It may be difficult to make a definitive diagnosis on a single 24-hour urinalysis; therefore repeated evaluation is often warranted. For example, it is desirable to confirm the presence of hypocitraturia or hyperuricosuria by repeat measurements. Table 46–5 Commercial Providers of 24-Hour Urinary Stone Risk Profiles Figure 46–3 Commercial 24-hour urine results are available and simplify the collection and reporting process. (Courtesy of Litholink, Inc.) Figure 46–4 The 24-hour urine results may be presented graphically to assist with interpretation and planning. (Courtesy of Mission Pharmacal, Inc.) There is controversy regarding the necessity of collecting two separate 24-hour urine specimens. As noted earlier, Rivers has advocated for the collection of two samples while on differing diets (random and restricted) (Rivers et al, 2000). Assuming that the patient complies, these data may be able to discern between type II absorptive hypercalciuria (AH II) and renal leak (hypercalciuria disappears while on the restricted diet in AH II). Researchers from Dallas have suggested that only one single 24-hour collection is required (Pak et al, 2001). Their study retrospectively reviewed and compared the results of two 24-hour urine samples that were collected on random diets. They noted no significant difference in the excretion of urinary calcium, oxalate, uric acid, citrate, pH, total volume, sodium, potassium, sulfate, or phosphorus. They concluded that the reproducibility of urinary stone risk factors was adequate in repeat samples, enough so that therapy would not have been altered. Conversely, Parks and colleagues (2002) have noted significant disparities between two separate collections. More than 1000 patients were examined from both private practice and academic settings. They noted that within nearly 70% of the comparisons, there were large enough differences such that the standard deviation would contain clinically relevant disparities. The authors therefore conclude that relying on one specimen alone could easily lead to misdiagnosis and, consequently, mismanagement. Key Points: Simplified Metabolic Evaluation Questions have arisen regarding the necessity of both chemical stone analysis and metabolic evaluation for patients with nephrolithiasis. Although stone composition analysis is neither always feasible nor desirable, there is helpful information from such an investigation that can aid with preventive therapy. Unfortunately, chemical and mineralogic names of common calculi are sometimes used interchangeably, causing significant confusion for the clinician. A list of these names is provided in Table 46–6. Table 46–6 Mineralogic Names of Renal Calculi Parks demonstrated that a given patient’s supersaturation of urinary crystals coincides with the stones produced by said patient (Parks et al, 1997). Indeed, in their study, treatments that reduced stone rates also reduced the supersaturation values of the historical stone composition for that patient. If calculi develop due to prolonged supersaturation of various crystals (e.g., calcium oxalate, urate), then it is further reassuring that “snapshot,” pooled urine supersaturation measurements accurately track stone admixtures and are a reliable index of long-term, “average” renal and urine supersaturations. Assessment of stone composition, not just urinary crystal supersaturation, can be a helpful adjunct to a metabolic evaluation. Because most stones are a mixture of more than one component, the relative ratios or predominance of any particular molecule may have predictive value. In an analysis of almost 1400 patients who had both stone analysis and a complete metabolic evaluation, Pak noted that calcium apatite and mixed calcium oxalate-calcium apatite stones were associated with the diagnoses of renal tubular acidosis and primary hyperparathyroidism (odds ratios ≥ 2) but not with chronic diarrheal syndromes (Pak et al, 2003b). As the phosphate content of the stone increased from calcium oxalate to mixed calcium oxalate-calcium apatite, and finally to calcium apatite, the percentage of patients with renal tubular acidosis increased from 5% to 39%, and those with primary hyperparathyroidism increased from 2% to 10%. Not surprisingly, pure and mixed uric acid stones were strongly associated with a gouty diathesis, whereas brushite stones were associated with renal tubular acidosis. As expected, there was a strong association between infection stones and infection and between cystine stones and cystinuria. These findings were further supported by Kourambas and colleagues (Kourambas et al, 2001). Stone composition correlated with metabolic findings in a series of 100 consecutive patients. A significant risk of renal tubular acidosis (RTA) was documented in those patients producing predominantly calcium phosphate calculi. They maintain that the finding of a noncalcareous stone simplifies the evaluation by focusing the ensuing workup on the most obvious cause. Pure uric calculi are primarily a result of gouty diathesis, and patients may not require further testing. Finally, Lingeman and colleagues noted that the finding of pure struvite/calcium apatite in a staghorn calculus predicted a low likelihood of finding other metabolic abnormalities during a workup. In their series, only 2 of 14 with pure infection stones had additional abnormalities compared with 7 of 7 patients with mixed chemical compositions. They therefore suggest that those patients with pure infection stones will not benefit from additional evaluation (Lingeman et al, 1995). Key Points: Use of Stone Analysis to Determine Metabolic Abnormalities Accompanying the ubiquity and increased utility of CT imaging, a number of authors have sought to identify characteristics wherein stone composition could be determined from this diagnostic modality (Mitcheson et al, 1983; Newhouse et al, 1984; Mostafavi et al, 1998; Nakada et al, 2000; Saw et al, 2000; Motley et al, 2001; Bellin et al, 2004; Deveci et al, 2004; Sheir et al, 2005). These investigations focused on Hounsfield unit (HU) measurements to determine stone composition. Both in vitro and in vivo work demonstrated significant differences in HU between pure uric acid stones and other stone types. However, it has been more difficult to differentiate pure struvite from cystine, calcium oxalate from brushite, and stones of mixed composition. Due to the significant variance in the readings obtained for different stone types, even with optimization of standard CT variables (collimation, pitch), this information has been of little clinical value. More recently, the application of dual energy CT (DECT) technology is demonstrating the potential to better characterize stone type. In vitro studies using ratios of HU during DECT have been able to distinguish between uric acid, calcium phosphate, and calcium oxalate calculi (Matlaga et al, 2008). Further discrimination of stone composition has been reported by two other sets of investigators. Boll and colleagues (2009), using an alternative calculation method, showed graphical separation of relatively pure cystine, struvite, calcium oxalate and calcium phosphate, and brushite stones. This group’s “DECTSlope” algorithm identified stone compositions unambiguously; however, separation of calcium oxalate and calcium phosphate calculi was not obtained. Finally, Grosjean and colleagues (2008) further characterized uric acid, cystine, struvite, calcium oxalate dihydrate, brushite, and calcium oxalate monohydrate into distinct groups with the use of DECT attenuation values. The ability to differentiate stone composition was noted to be lost when the image was subjected to respiratory motion artifact. Early work with DECT is promising but must be confirmed in vivo before incorporation into clinical decision making for the metabolic evaluation and medical management of nephrolithiasis. Please see the Expert Consult website The costs associated with the treatment of nephrolithiasis are undoubtedly substantial. In 1984 Shuster and Scheaffer (1984) estimated that the average stone episode cost approximately $2000, exclusive of recurrences. At the time this finding was based on a predominance of open surgical approaches with an average hospital stay lasting 4 to 5 days. The average annual cost of recurrence for a current stone case was conservatively estimated to be in the $300 to $400 range. Based on these conservative projections, they estimated that the entire national population of white males in the age range of 18 to 60 years yielded an annual cost due to kidney stones approaching $315,000,000 (Shuster and Scheaffer, 1984). By 1993, the estimated costs continued to climb, despite advances in technology and decreases in inpatient care. Indeed, Clark and colleagues performed a review of prevalence data for urolithiasis and the relative frequency of surgical treatments from Civilian Health and Medical Program of the Uniformed Services claims data. They found that the total charges for evaluation, hospitalization, and treatment were estimated to be $1.23 billion per year. Professional charges for those who were hospitalized were estimated to be $183 million. Outpatient evaluation of urolithiasis was expected to cost $278 million. Indirect costs for lost wages were estimated to be $139 million. This totaled a staggering annual cost of $1.83 billion in the United States alone (Clark et al, 1995). At the onset of the new millennium, the economic burden of urolithiasis continued to rise. Estimations of the annual medical expenditures for stone disease in the United States for 2000 were $2.1 billion, inclusive of $971 million for inpatient services, $607 million for physician office and hospital-based outpatient services, and $490 million for emergency department charges (Pearle et al, 2005). These calculations are based on a range of nationally available datasets and do not necessarily reflect the additional societal costs of lost productivity and social service support. These costs are clearly not negligible because the peak incidence of urolithiasis occurs in patients between 20 and 60 years old (the years of highest workers’ productivity), and an analysis of more than 300,000 beneficiaries from 25 large U.S. employers identified that 30% of urolithiasis patients missed an average of 19 hours of work and had an additional $3500 in annual medical costs (Saigal et al, 2005). With the increasing incidence of stone disease, one can only conclude that the national health care expenditure for urolithiasis will continue to rise. Particularly worrisome is the increasing evidence that obesity confers an increased risk of nephrolithiasis is quite sobering considering the epidemic of obesity that is enveloping the United States (Curhan et al, 1998a; Ekeruo et al, 2004; Morrill and Chinn, 2004; Rigby et al, 2004; Strumpf, 2004; Taylor et al, 2005a). With these figures in mind, prudence would dictate that medical prevention could help curb runaway costs and prevent long-term sequelae of recurrent nephrolithiasis. As an example, Madore has found an association between nephrolithiasis and the eventual development of hypertension (Madore et al, 1998a, 1998b). This ubiquitous condition has its own attendant costs and risk of end-organ damage such as cardiovascular disease and renal failure. Because these subsequent disorders have their own costs and risks, any reasonable chance to break the chain of events should be sought. The emergence and instant appeal of shock wave lithotripsy and improved endoscopy in the mid-1980s prompted some authors to remind the urologic community that medical assessment was still a viable option (Resnick and Pak, 1987; Preminger, 1994). However, office visits, serum studies and 24-hour urine studies have their own costs. Is there a break-even point at which the costs of a metabolic evaluation, pharmacologic prophylaxis, and continued office visits are less than the expense of surgical management? Chandhoke compared the cost of medical prophylaxis with the cost of clinically managing recurrent stone episodes (Chandhoke, 2002). Additionally, he determined the stone recurrence rate without prophylaxis (stone frequency) at which these two treatment approaches became cost equivalent. This review conducted a cost survey in 10 countries to compare costs of medical prophylaxis and managing recurrent acute stone episodes. Costs of an acute stone episode included an emergency department visit, associated radiographic imaging to confirm diagnosis of a symptomatic stone, and outpatient treatment of upper urinary tract stones that did not pass spontaneously. Costs of medical management included an initial limited metabolic evaluation, drug therapy, a follow-up office visit every 6 months that included a 24-hour urinalysis, and radiographic imaging of the kidneys, ureters, and bladder once a year. Not surprisingly, the costs of medical prophylaxis and managing an acute stone episode varied significantly from country to country. The stone frequency at which costs of these management options became equivalent ranged from 0.3 to 4 stone episodes a year. This study concluded that medical management of a first stone episode is not cost-effective and that individual decisions should be determined by local costs. Researchers at the University of Texas, Southwestern Medical Center have created a decision tree model to evaluate the cost-effectiveness and stone recurrence rates of common management strategies in stone formers (Lotan et al, 2004). They evaluated six common medical strategies, namely dietary measures alone (conservative), empiric drug treatment (empiric), or directed drug therapy on the basis of a simple or comprehensive metabolic evaluation. The model made reasonable assumptions regarding costs for evaluation, medications, emergency treatment, and surgery for stone recurrence. A review of the literature guided estimations of stone recurrence and risk reduction from various medical therapies. They found that first-time stone formers were best treated with a conservative approach because it was the least costly and it yielded a stone formation rate of 0.07 stones per patient yearly. For recurrent stone formers, conservative treatment was less costly than drug treatments, but it was associated with a higher stone recurrence rate (0.3 stones per patient yearly). Directed medical therapies were more costly than conservative treatment ($885 to $1187 vs. $258 yearly), but they provided the obvious advantage of decreasing recurrence rates by 60% to 86%. Key Points: Economics of Metabolic Evaluation Using an ambulatory protocol, the etiology of nephrolithiasis can be classified into 12 separate categories reflecting specific physiologic derangements. The details regarding the physiology and pathophysiology of these distinct entities are included in Chapter 45. These categories are listed in Table 46–7 along with the relative frequency of their occurrence as noted by Pak and colleagues at a dedicated Stone Clinic in an academic medical center (Levy et al, 1995). An argument can be made that these relative incidences may not be representative of the general population for two reasons. First, referral to an academic center may imply a more serious version of stone disease and may therefore represent a selection bias. Secondly, recognizing that there are at least some regional variations of stone incidence (Harvey et al, 1990), this particular patient population may be distinctly different from those in a different region of the United States or other regions of the world. Table 46–7 Classification of Nephrolithiasis Modifed from Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med 1995;98(1):50–9. Renal hypercalciuria (RH) (also known as renal leak hypercalciuria) is thought to be due to a wasting of calcium by the functioning nephron. The details of this process and various hypotheses are outlined in Chapter 45. As a result of constant loss of calcium from the distal tubules, these patients will demonstrate hypercalciuria during all phases of fasting, loading, or restriction of dietary calcium. Most patients with RH will have normal serum calcium but may exhibit a mild elevation of iPTH as the regulatory systems attempt to “keep up” with the constant loss of calcium. Patients with this disorder suffer from an overproduction of parathyroid hormone from either one dominant adenoma or from diffuse hyperplasia of all four glands. The hallmark of this disorder is the persistence of increased urinary calcium during all parts of the dietary calcium manipulations. In addition, these patients frequently demonstrate hypercalcemia and elevations of the parathyroid hormone. The measurement of only the intact portion of the hormone (iPTH) has avoided confusion from the measurement of fragments of the same molecule (Kao et al, 1982; Nussbaum et al, 1987) and has greatly enhanced the ability to make this diagnosis. Unfortunately, some patients may have normocalcemic hyperparathyroidism. These patients may be difficult to distinguish from those with renal leak hypercalciuria, during which serum calcium will be normal but a mild elevation of the iPTH can occur, creating a secondary hyperparathyroidism. In these instances the patients can be treated with a 2-week course of a thiazide diuretic such as chlorthaladone 25 mg daily. If the patient actually suffers from renal leak, than the calcium loss should be suppressed and the iPTH should return to normal (Aroldi et al, 1979; Barilla and Pak, 1979; Zechner et al, 1981). Those with true primary hyperparathyroidism will continue to circulate elevated levels of iPTH and may become mildly hypercalcemic, although this latter feature has been debated in the literature (Klimiuk et al, 1981; Farquhar et al, 1990; Strong et al, 1991). Idiopathic hypercalciuria can be found in both normal people and among stone formers (Coe et al, 1979). These patients may demonstrate elevated amounts of urine calcium in all phases of the dietary calcium manipulation but will not demonstrate serum abnormalities. On a cautionary note, this term does not always enjoy a strict definition and is sometimes substituted to describe those patients with hypercalciuria who have not undergone further evaluation to discriminate between the various subcategories. Although this diagnosis is not as “clean” as possible, it represents a more pragmatic approach to hypercalciuria because the treatment for absorptive and renal hypercalciuria is often the same (as outlined later in this chapter). Table 46–8 summarizes the laboratory parameters that help delineate the various types of hypercalciuria. Patients with hyperuricosuria may be prone to the formation of calcium oxalate calculi through a process called heterogeneous nucleation (also referred to as epitaxy) (Coe and Kavalach, 1974; Pak and Arnold, 1975; Coe, 1980). The details of this process are outlined in the previous chapter. These patients give a history of calcium oxalate nephrolithiasis and may have a history of hyperuricemia with symptomatic gout. During metabolic evaluation, these patients will demonstrate hyperuricosuria (>800 mg/day). This entity is often one of the most striking findings during a metabolic evaluation because it involves multiple factors, all caused as a result of chronic diarrhea with its attendant dehydration and bicarbonate losses (Worcester, 2002). The main hallmark is, of course, hyperoxaluria with values that can be quite high (i.e., >50 mg/day). As a result of intestinal fluid loss, patients will often exhibit low urine volumes. The bicarbonate loss (and the consumption of citrate as an acid/base buffer) can also cause a low urine pH and hypocitraturia (Rudman et al, 1980). Urine calcium excretion is often low due to the saponification of oral calcium with poorly absorbed fats in the intestinal tract. This extremely rare disorder is caused by an inborn error of metabolism. The more common variant, type 1, is due to a defect of the enzyme alanine-glyoxylate aminotransferase (AGT) via an autosomal recessive inheritance. Type 2 is a less common variant thought secondary to a defect in D-glycerate dehydrogenase, which has both glyoxylate/hydroxypyruvate reductase. Primary hyperoxaluria usually present during childhood with early stone formation, tissue deposition of oxalate (oxalosis), and renal failure due to nephrocalcinosis. Death often occurs before age 20 in untreated patients (Williams and Smith, 1968; Leumann and Hoppe, 1999). Metabolic evaluation will reveal high urine oxalate excretion, as well as high serum levels of this molecule. The importance of dietary oxalate and the possibility of an inheritable sensitivity to oral oxalate loads are debated and have already been discussed in the previous chapter. It appears increasingly evident that a deficiency of a bacterium found within intestinal flora (Oxalobacter formigenes) is a factor in the formation of calcium oxalate calculi (Allison et al, 1986; Sidhu et al, 1999; Troxel et al, 2003). Regardless of the underlying etiology, some patients without primary hyperoxaluria or without a history of bowel disorders will demonstrate an elevation of oxalate in their 24-hour urine collection. A review of the patient’s dietary habits may reveal a predisposition for those foods that are particularly high in oxalate. Although this molecule is ubiquitous and cannot be avoided, certain foods can deliver substantial amounts of oxalate in one serving. An abbreviated list of foods that are particularly high in oxalate are contained in Table 46–9 (Assimos and Holmes, 2000; Holmes and Assimos, 2004).
Diagnostic Evaluation of Nephrolithiasis
Selection of Patients for Metabolic Evaluation
First-Time Stone Formers
Abbreviated Protocol for Low-Risk, Single Stone Formers
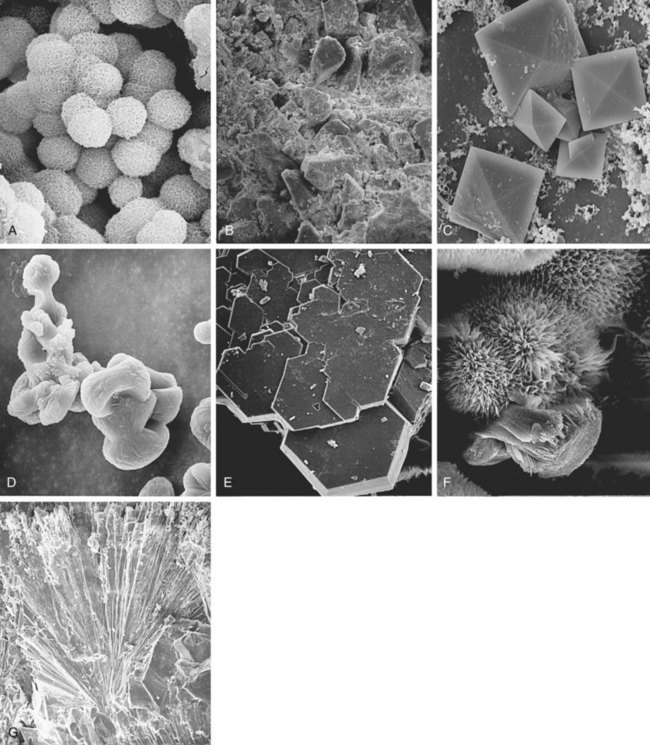
CHEMICAL TYPE
APPEARANCE
Calcium oxalate monohydrate
Hourglass
Calcium oxalate dihydrate
Envelope, tetrahedral
Calcium phosphate-apatite
Amorphous
Brushite
Needle shaped
Magnesium ammonium phosphate (struvite)
Rectangular, coffin-lid
Cystine
Hexagonal
Uric acid
Amorphous shards, plates
Extensive Diagnostic Evaluation
Fast and Calcium Load Test
Simplified Metabolic Evaluation
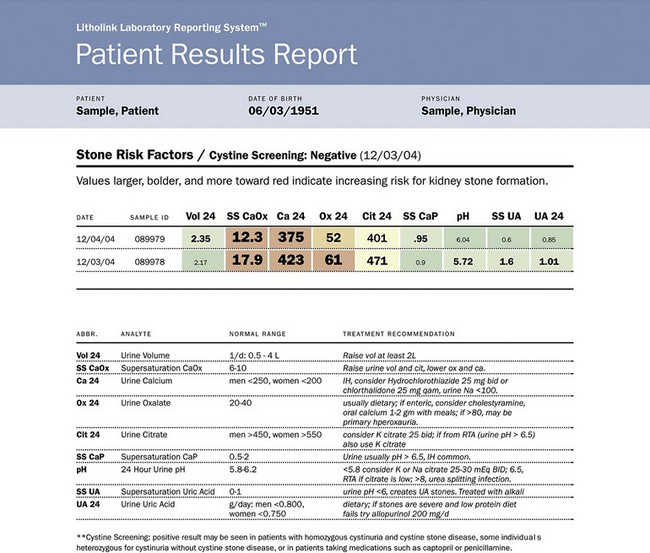
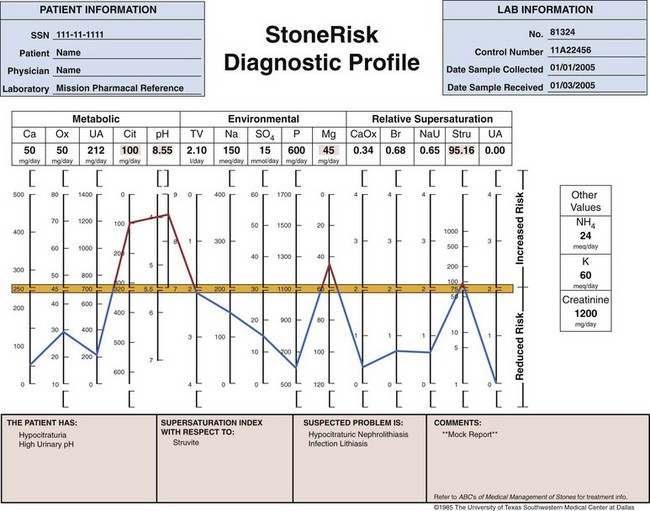
Use of Stone Analysis to Determine Metabolic Abnormalities
Calcium oxalate monohydrate
Whewellite
Calcium oxalate dihydrate
Weddellite
Calcium hydrogen phosphate dihydrate
Brushite
Tricalcium phosphate
Whitlockite
Carbonate-apatite
Carbonate-apatite
Magnesium ammonium phosphate
Struvite
Cystine
None
Uric acid
None
Role of Imaging in Determining Stone Composition
Economics of Metabolic Evaluation
![]() for this section.
for this section.
Classification of Nephrolithiasis and Diagnostic Criteria
Percentage
SOLE OCCURRENCE
COMBINED OCCURRENCE
Absorptive hypercalciuria
20
40
Type I
Type II
Renal hypercalciuria
5
8
Primary hyperparathyroidism
3
8
Unclassified calcium nephrolithiasis
15
25
Hyperoxaluric calcium nephrolithiasis
2
15
Enteric hyperoxaluria
Primary hyperoxaluria
Dietary hyperoxaluria
Hypocitraturic calcium nephrolithiasis
10
50
Distal renal tubular acidosis
Chronic diarrheal syndrome
Thiazide-induced
Idiopathic
Hypomagnesiuric calcium nephrolithiasis
5
10
Gouty diathesis
15
30
Cystinuria
<1
Infection stones
1
5
Low urine volume
10
50
No disturbance and miscellaneous
<3
Calcium-Based Calculi
Hypercalciuria (>200 mg/day)
Renal Hypercalciuria
Resorptive Hypercalciuria (Primary Hyperparathyroidism)
Idiopathic Hypercalciuria
Hyperuricosuric Calcium Nephrolithiasis
Hyperoxaluria (>40 mg/day)
Enteric Hyperoxaluria
Primary Hyperoxaluria
Mild Metabolic Hyperoxaluria (Dietary)
Evaluation and Medical Management of Urinary Lithiasis
• Routine performance of a comprehensive metabolic evaluation may not be economically sound if applied to all stone patients.