Arthur L. Burnett, MD, MBA, FACS
Historical Perspective
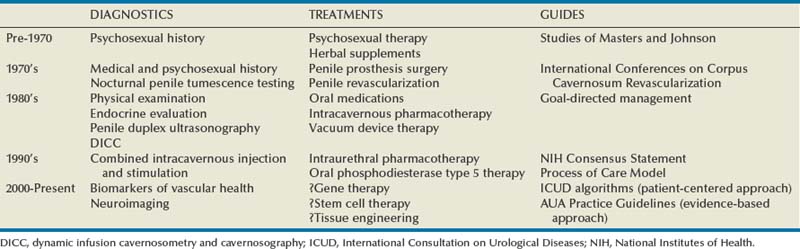
Over just the past quarter century, the clinical management of erectile dysfunction (ED) has changed remarkably (Table 24–1). This comment relates to the success of greatly improved therapeutic options in the field, well demonstrated by oral phosphodiesterase type 5 (PDE5) inhibitor pharmacotherapy. Previously, ED management was mostly empirical and frequently used options were psychoanalysis, sex therapy, and endocrine treatments. Other notional prescriptions ranged from aphrodisiacs and erection enhancement pills to penile rigidity-inducing devices and surgically implanted penile prosthetics.
Besides advances in its therapeutic dimension, the field emerged overall as a recognized clinical discipline. Additional highlights in this regard included reaffirmations of its nosological foundations and its principles of practice for the patient with ED. Physiologic penile erection and its impairment were described within the context of the male sexual response cycle, and the historically pejorative and ambiguous term “impotence” was replaced with the more euphemistic and well-defined term “erectile dysfunction.” Along the way, the importance of the patient’s subjective claim of the existence of the problem was fully recognized, with emphasis given to the roles of both the patient and partner in its evaluation and management. Lue originally proposed the concept of the “goal-directed approach” for the management of ED, recognizing that lesser-invasive, reversible therapeutic methods had become available by the 1980s that did not require extensive and costly diagnostic testing before their implementation (Lue, 1990). The approach pronounced the imperative role of the clinician in performing a standard evaluation on the basis of a thorough and adequate sexual, medical, and psychosocial history in combination with a focused physical examination and select laboratory testing. However, it also acknowledged that diagnostic and therapeutic decisions should rely on the goals and preferences of the patient (and partner). This manner emphasizes the “patient-centered” intent brought to ED management (Rosen et al, 2004c).
The clinician’s responsibility in recognizing and managing ED, as for all sexual dysfunctions, has gained the support of many thought leaders in the field of sexual medicine. Guidelines for the management of sexual dysfunctions with worldwide acceptance have resulted from numerous consensus meetings. Prominent among these were the series of International Consultations on Sexual Medicine (ICSM), cosponsored variously by the World Health Organization, International Consultation on Urological Diseases, American Urological Association, Société Internationale d’Urologie, and the International Society for Sexual Medicine, the first held in July 1999 with subsequent conferences convened in July 2004 and in July 2009 (Jardin et al, 2000; Lue et al, 2004; Montorsi et al, 2010). Published proceedings from these congresses established diagnostic and therapeutic algorithms, which were founded on the principles of the goal-directed clinical management approach and obliged the trained clinician/physician to assess the patient and couple completely and prescribe therapy appropriately. They have also proclaimed that sexual medicine should be practiced in accordance with the highest standards of ethics, quality, safety and cost-effectiveness.
Public Health Significance
Epidemiology
Epidemiologic investigation, which specifies that study results are readily generalized to the overall male population, has provided powerful information regarding the nature, etiology, and prognostic ramifications of ED. The most thoroughly studied sexual dysfunction in the context of epidemiologic research, ED is estimated to carry an overall adult male (older than 20 years of age) prevalence rate of 10% to 20% worldwide, with the majority of studies reporting a rate closer to 20% (Derogatis and Burnett, 2008). It was estimated that there were more than 152 million men worldwide who experienced ED in 1995, with a projection of the prevalence reaching approximately 322 million men having ED by 2025 (Aytac et al, 1999). This trend is maintained irrespective of racial/ethnic background or geographic region. Current data have also confirmed that the prevalence of ED mounts with increasing age and the presence of comorbid medical conditions, which include type 2 diabetes mellitus, obesity, cardiovascular disease, hypertension, dyslipidemia, depression, and prostate disease/benign prostatic hypertrophy (BPH) (Braun et al, 2000; Martin-Morales et al, 2001; Nicolosi et al, 2004; Rosen et al, 2004b; Saigal et al, 2006; Laumann et al, 2007; Selvin et al, 2007). This correlation has supported the premise that ED and comorbid medical conditions share pathophysiologic mechanisms such as endothelial dysfunction, arterial occlusion, and systemic inflammation (Solomon et al, 2003; Montorsi et al, 2004; Billups, 2005; Ganz, 2005; Kloner, 2005; Guay, 2007).
Although they are few in number, prospectively conducted longitudinal studies have documented the true incidence and disease risk relationships for ED. In one study, a crude ED incidence rate was 25.9 cases/1000 man-years among men aged 40 to 69 years (Johannes et al, 2000). According to another study, incident ED statistics were 57% at 5 years and 65% at 7 years in men 55 years or older (Thompson et al, 2005). Such studies have uniquely affirmed predictors for the development of ED, which include age, lower education, diabetes, cardiovascular disease, hypertension, cigarette smoking, cigar smoking, passive exposure to cigarette smoke, and overweight condition (Feldman et al, 2000; Johannes et al, 2000; Inman et al, 2009).
However, the strength of the risk association is also gauged from the opposite analytic direction, and incident ED may indeed inform the risk of subsequent disease morbidity and mortality. This relationship has been best demonstrated so far with respect to cardiovascular disease. The placebo arm of the Prostate Cancer Prevention Trial found that ED is a sentinel for future risk of cardiovascular events, comparable with that of current cigarette smoking or a family history of myocardial infarction (Thompson et al, 2005). This study established that men with ED were 45% more likely than men without ED to experience a cardiac event after 5 years of follow-up (Thompson et al, 2005). In another population-based study of community-dwelling men followed longitudinally, ED was associated with an approximately 80% higher risk of subsequent coronary artery disease at 10 years (Inman et al, 2009). In a long-term follow-up (15 years) of the Massachusetts Male Aging Study (Feldman et al, 1994), ED was found to be positively associated with subsequent all-cause and cardiovascular disease mortality and constituted a risk in this regard similar to that of conventional risk factors such as increased body mass index, diabetes, and hypertension (Araujo et al, 2009). These compelling data, considered alongside results generated from multiple smaller clinical cohort studies in the field, have contributed profoundly toward the understanding that the diagnosis of ED represents a clinical barometer of overall male health status and further serves to catalyze efforts to prevent disease, promote health and, moreover, improve survival.
Health Policy
Sexual dysfunctions and ED specifically have taken on increasing importance with respect to their socioeconomic impact. Besides its medical comorbidity associations, ED is recognized to adversely affect quality of life, decrease occupational productivity, and increase health care resource utilization (Krane et al, 1989; Litwin et al, 1998). Because of the heightened ease of use and availability of effective first-line treatments combined with a growing societal awareness of ED and acceptance of its treatment, it is understandable that a trend toward increased health care services utilization surrounding ED has been observed (Wessells et al, 2007).
ED can be included among a host of urologic diseases having a substantial burden on the public financially. Total expenditures for outpatient clinical management of ED (exclusive of pharmaceutical costs) in the United States in 2000 approximated $330 million, ranking ninth most costly among most frequent urologic diagnoses (Litwin et al, 2005). By contrast, this cost was approximately $185 million in 1994 (Wessells et al, 2007). Individual-level expenditures on an annual basis associated with an ED diagnosis (inclusive of pharmaceutical costs) among affected 18- to 64-year-old males in the United States in 2002 were calculated to be $1107 (Wessells et al, 2007). These data have enormous implications for governmental, as well as nongovernmental agencies in the United States and worldwide, whose work must consider the practical distribution and fiscal allocation of health care services for ED.
Key Points
Epidemiology and Health Policy
Management Principles
Early Detection
Epidemiologic and clinical investigation has suggested that many patients with ED retain adverse clinical conditions and also lifestyle factors (e.g., diabetes, cardiovascular disease, prostate disease, overweight condition, current cigarette smoking, physical inactivity) that potentially compromise erectile function (Saigal et al, 2006; Laumann et al, 2007; Selvin et al, 2007). It is estimated that as much as 75% of men with diabetes have ED to some degree (Hakim and Goldstein, 1996). Similar rates of ED diagnoses are described for men presenting clinically with other chronic diseases, which constitute risk factors for ED (Jackson, 1999; Burchardt et al, 2000; Montorsi et al, 2003b; Solomon et al, 2003).
In addition to adverse health conditions having risk associations with ED, medication use has also been associated with ED in up to 25% of presentations (Keene and Davies, 1999; Francis et al, 2007). The most commonly implicated classes of drug include antihypertensive drugs such as thiazide diuretics and β-adrenoceptor antagonists and psychotherapeutic drugs, particularly selective serotonin reuptake inhibitor (SSRI) antidepressants. Table 24–2 lists several drug classes commonly associated with ED. It is importantly recognized that medications may affect other components of the male sexual response cycle including sexual desire, arousal, and orgasm, which secondarily hampers erectile function. Of additional importance, the assignment of causation of ED for any particular medication is conditional, requiring that an increased prevalence exists in the target population compared with the placebo group after stratification for known risk factors or compared with another drug with an equivalent therapeutic effect, and further, a credible physiologic mechanism should be established experimentally (Sáenz de Tejada et al, 2005).
Table 24–2 Drugs Associated with Erectile Dysfunction
| CLASS | SPECIFIC AGENTS |
|---|---|
| Antihypertensives | Thiazide diuretics, nonselective β-blockers |
| Antidepressants | Tricyclics; selective serotonin reuptake inhibitors |
| Antipsychotics | Phenothiazines |
| Antiandrogens | Nonsteroidal (flutamide); steroidal (cyproterone acetate); luteinizing hormone-releasing hormone analogues |
| Antiulcer drugs | Histamine H2 receptor antagonists (cimetidine) |
| Cytotoxic agents | Cyclophosphamide, methotrexate |
| Opiates | Morphine |
Calculated odds ratios underscore the extent to which various ED risk factors correlate with ED (Table 24–3). These data support the contention that patients with identifiable ED risk factors likely experience the sexual dysfunction currently or will eventually develop it at some time. Clinical screening of such patients based on these indications may allow advantageous opportunities to diagnose and treat ED.
Table 24–3 Major Erectile Dysfunction Risk Factors
| CONDITION | MULTIVARIATE ADJUSTED ODDS RATIO |
|---|---|
| Diabetes mellitus | 2.9 |
| Hypertension | 1.6 |
| Cardiovascular disease | 1.1 |
| Hypercholesterolemia | 1.0 |
| Benign prostate enlargement | 1.6 |
| Obstructive urinary symptoms | 2.2 |
| Increased body mass index (>30 kg/m2) | 1.5 |
| Physical inactivity | 1.5 |
| Current cigarette smoking | 1.6 |
| Antidepressant use | 9.1 |
| Antihypertensive use | 4.0 |
From Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007;120:151–57; and Francis ME, Kusek JW, Nyberg LM, Eggers PW. The contribution of common medical conditions and drug exposures to erectile dysfunction in adult males. J Urol 2007;178:591–6.
Goal-Directed Management
A goal-directed approach to the management of patients with ED has largely been practiced in the field over the past two decades since Lue’s original description (Lue, 1990). The approach dictates that the diagnostic evaluation and therapeutic plan relates to the individual patient’s presentation and manner of deriving satisfaction, in accordance with a patient-centered framework (Hatzichristou et al, 2010). The basic aim of goal-directed management is to allow the patient or couple to make an informed selection of the preferred therapy for sexual fulfillment on the basis of a sound understanding of all treatment options after completing a thorough discussion with the treating clinician. The approach recognizes that patients vary in their acceptance of their sexual disorders and in their interest to pursue management. Their decisions accordingly follow individual preferences, needs, and expectations regarding management options. Evaluations of this approach have affirmed its utility and demonstrated that patient therapeutic preferences accord with the least invasive forms of therapy (Jarow et al, 1996; Hanash, 1997).
Role of Partner Interview
The partner interview is a critical component in initiating management of ED. Partner interviews have been shown to impact diagnosis and treatment in up to 58% of cases (Tiefer and Schuetz-Mueller, 1995; Chun and Carson, 2001). The partner may be the source of important information that guides optimal intervention and response to therapy. The partner may share a new and different perspective on sexual issues affecting the couple, provide insight into the quality of the couple’s relationship, and relate his/her role in the sexual dysfunction (Speckens et al, 1995; Fisher et al, 2009). The partner’s involvement and attitude may also affect the patient’s initiation of and adherence to therapy (Jackson and Lue, 1998; Fisher et al, 2005).
An important additional consideration is that partners’ well-beings may be affected by the patients’ ED conditions. Studies have shown that women partners of men with ED are themselves more likely to have sexual dysfunction or to cease sexual activity entirely (Ichikawa et al, 2004; Montorsi and Althof, 2004; Fisher et al, 2005; Sand and Fisher, 2007). This observation further prompts the facilitatory role of the partner in ED management, which maximizes the success of therapy and inherently satisfaction of the couple.
In practice, additional office visits as needed, in which the partner accompanies the patient, and the communication of educational information to the partner via the patient are recommended techniques for involving partners in ED management (Dean et al, 2008).
Cardiac Risk Assessment
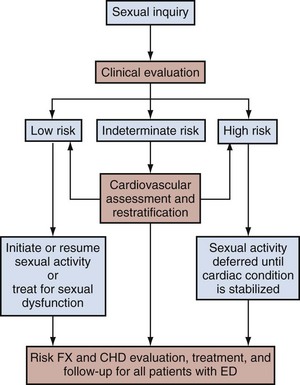
The frequent coexistence of ED and cardiovascular disease, as established by clinical epidemiologic study and by basic science research, has steered ED management to include procedures that account for the ED patient’s cardiovascular health risks. The second Princeton Consensus Guidelines Panel reinforced the linkage between sexual activity and cardiac risk, which was acknowledged with the first conference (DeBusk et al, 2000), and pronounced that all men with ED, even in the absence of manifesting cardiac symptoms, should be regarded as having potential risks for cardiovascular disease (Kostis et al, 2005; Jackson et al, 2006).
ED patients are recommended to undergo a full medical assessment with stratification of cardiovascular risk as high, medium, or low (Fig. 24–1). Patients classified as having high risk would be those with unstable or refractory angina, a recent history of myocardial infarction, certain arrhythmias, or uncontrolled hypertension. For these patients, sexual activity with any particular ED therapy should be deferred until the cardiac condition is stabilized. Such patients should ideally undergo cardiologic referral for cardiovascular stress testing and subsequent risk reduction therapy. Importantly, even patients at low risk for cardiovascular events should receive the minimum recommendations of cardiovascular disease management. Basic intervention includes counseling for lifestyle modifications such as increased physical activity and improved weight control combined with regular health monitoring by the patient’s general practitioner (Kostis et al, 2005).
Step-Care Approach
Practitioners of ED management have always sought a rational approach for implementing diagnostic and therapeutic options. The “Process of Care Model for Erectile Dysfunction” was proposed as a stepwise methodology, combining processes, actions, and outcomes in the management of the ED patient (Process of Care Consensus Panel, 1999). It specified an algorithm for therapeutic decision making that takes into account patient needs and preferences (goal-directed management), although it was also based on specific criteria such as ease of administration, reversibility, relative invasiveness, and cost of therapies. This algorithm presented a strategy of staged therapy (i.e., first-, second-, and third-line interventions), which ranged from lifestyle modification to surgery. In concept, the scheme has been borrowed and endorsed by other consensus panels, which acknowledged the purpose of patient education and counseling along with medical therapies as initial forms of ED management in common practice (Montague et al, 2005; Hatzichristou et al, 2010).
Specialist Referral
The advent of effective oral pharmacotherapy for ED has recently enabled many primary practitioners to feel comfortable with managing the majority of clinical presentations of ED. At the same time, it is understood that situations arise in which the patient or primary practitioner may request the assistance of a consultant/specialist (e.g., cardiologist, endocrinologist, psychologist, urologist) for further diagnostic evaluation and treatment beyond the boundaries of initial management (Process of Care Consensus Panel, 1999). Such referrals may be required for individuals with complicated or atypical presentations of ED, representing diagnostic challenges that exceed common clinical practices of nonspecialists. Specialized evaluation and management potentially offer improved therapeutic outcomes for these presentations.
Follow-up Care
Follow-up care is an essential part of ED management and should not be overlooked. The objectives of this action are manifold. A primary basis is to ensure continual success with the therapeutic outcome. It has been shown that treatment discontinuation occurs at high rates among patients who are not reassessed regularly (Albaugh et al, 2002). Additional purposes are to reassess medical and psychosocial conditions adversely impacting ED and success of therapy, evaluate the need for dosage titration or treatment substitution, and monitor adverse drug interactions or drug interaction effects. As always, follow-up attention offers educational opportunities for patient and partner with regard to addressing sexual health concerns, as well as lending guidance for related health care matters.
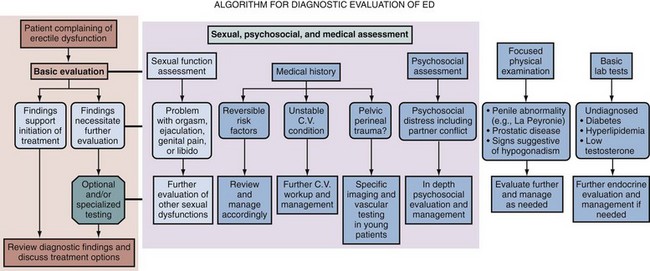
Diagnostic Evaluation
The cornerstone in the evaluation of ED involves a detailed case history, preferably taken from patient and partner, physical examination, and proper laboratory tests (Fig. 24–2). The diagnosis can be submitted on the basis of an individual’s report of consistent inability to attain and maintain an erection of the penis sufficient to permit satisfactory sexual intercourse (NIH Consensus Statement, 1992; Lewis et al, 2004). It is noteworthy that the original National Institutes of Health definition did not specify a parameter for the duration of symptoms to accept the diagnosis. Subsequent organizational statements did apply a 3-month interval as a minimal requirement diagnostically, except for cases of trauma or surgically induced ED (Lewis et al, 2004).
Sexual, Medical, and Psychosocial History
The comprehensive assessment of any sexual problem begins with the performance of a detailed case history including sexual, medical, and psychosocial components. The clinician may employ brief checklists or questionnaires for the purpose of recognizing the problem and initiating its evaluation, although he or she should standardly perform a detailed interview to understand the nature of the sexual complaint. The sexual history component in particular should be elicited with utmost sensitivity, given the intrapersonal and interpersonal aspects of sexual dysfunction (Rosen et al, 2004c). Additional emphasis has recently been given to providing cultural competence when interacting with patients (Hatzichristou et al, 2010). All discussion of sexual matters is done privately and confidentially, and the clinician is required to express trust and concern, as well as a nonjudgmental manner that epitomizes the doctor-patient relationship. The clinician should not assume that every patient is involved in a monogamous, heterosexual relationship. However, the situation may be presented whereby the partner can be interviewed, and this opportunity may be used, with the approval of the patient, to corroborate aspects of the clinical history and confirm mutual therapeutic goals.
Sexual History
The potential etiology of ED is commonly probed and may be categorized as psychogenic, organic, or mixed according to whether there is a presumed psychologic or interpersonal determinant (psychogenic); a specific endocrinologic, neurologic, or cardiovascular cause (organic); or coexistence of psychologic or relationship factors and organic causes (mixed) (Table 24–4) (Ralph and McNicholas, 2000). It is accepted that ED many times cannot be fully dichotomized into psychogenic and organic categories. However, its characterization by a predominant etiologic basis may nonetheless assist therapeutic objectives. The interview should also assess whether ED is the primary source of the presenting complaint or secondary to some other aspect of the sexual response cycle (e.g., desire, ejaculation, orgasm) that may also relate to the clinical presentation (Rosen et al, 2004c). The association of decreased arousal, if present, may be explored as well and evaluated as to whether it preceded or was incidental to the development of ED.
Table 24–4 Classification of Erectile Dysfunction
| PSYCHOGENIC | ORGANIC |
|---|---|
| Sudden onset | Gradual onset |
| Complete immediate loss | Incremental progression |
| Situational dysfunction | Global dysfunction |
| Waking erections present | Waking erections poor/absent |
Adapted from Ralph D, McNicholas T. UK management guidelines for erectile dysfunction. BMJ 2000;321:499–503.
Questionnaires and Sexual Function Symptom Scores
Self-administered ED questionnaires are extremely useful adjuncts to the case history, and they concur with the patient’s self-report in establishing the diagnosis. Early supplied questionnaires in the field such as the Derogatis Sexual Function Inventory (245 items) (Derogatis and Melisaratos, 1979) and the Golombok Rust Inventory of Sexual Satisfaction (GRISS) (28 items) (Rust and Golombok, 1986) were detailed, and they commonly aimed to differentiate psychogenic and nonpsychogenic ED or evaluate sexual functioning in the context of the couple. More recently developed instruments were implemented primarily in clinical trials associated with new drug development, and they captured particular efficacy end points including sexual interest, performance, and satisfaction. However, as part of practice pattern shifts that have occurred in ED management in recent years, there has been a growing emphasis on and application of patient self-reported instruments for clinical practice. These self-report measures have been meant to be brief and practical and to serve in documenting the presence, severity, and responsiveness to treatment of ED.
The most widely referenced instruments include the International Index of Erectile Function (IIEF) by Rosen and colleagues (1997), the Brief Male Sexual Function Inventory (BMSFI) by O’Leary and colleagues (1995), the Center for Marital and Sexual Health Sexual Functioning Questionnaire by Glick and colleagues (1997), the Changes in Sexual Functioning Questionnaire by Clayton and colleagues (1997), and the Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) by Althof and colleagues (1999). The IIEF, which contains 15 items that address and quantify five domains—erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction—is the most widely used questionnaire (Fig. 24–3). An abridged five-item version of this instrument, the IIEF-5, has been useful to clinicians in routine clinical practice specifically for the evaluation of ED (Rosen et al, 1999). The instrument classifies ED severity into five categories: severe (5 to 7), moderate (8 to 11), mild to moderate (12 to 16), mild (17 to 21), and no ED (22 to 25). The Male Sexual Health Questionnaire offers another instrument that assesses core components of male sexual function (i.e., desire, erection, ejaculation, satisfaction) and has utility in both clinical and research settings (Rosen et al, 2004a). The Sexual Experience Questionnaire has also recently been developed as a tool for evaluating health-related quality of life concepts and comprises erection, individual satisfaction, and couple satisfaction domains (Mulhall et al, 2008).
A known limitation of self-administered questionnaires is that they do not distinguish an etiologic basis for ED, that is, they do not differentiate among the various causes of ED (Blander et al, 1999; Kassouf and Carrier, 2003). Further, they may not sufficiently indicate the severity of ED that is evidenced on objective grounds (Tokatli et al, 2006). Although the exact nature of the ED diagnosis arguably is not absolutely necessary to initiate ED treatment today with current management options, it is understood that further clinical evaluation with diagnostic tests may be required to discern the basis and extent of the ED by system (e.g., vascular, neurologic, endocrinologic) and take action that may be most effective and possibly corrective.
Specialized Evaluation and Testing
The implicit goal of specialized evaluations in medicine in general is to improve diagnostic accuracy and direct successful therapy on the basis of the specific diagnosis. A similar principle applies to sexual medicine. However, at the present time, despite the availability of various technologies that may specify and define the causation for ED (i.e., vasculogenic, neurogenic, endocrinogenic, psychogenic), the treatment plan for this sexual dysfunction can often be formulated without carrying out extensive diagnostic testing. Nonetheless, such testing is frequently applied for diagnostic precision, typically by specialists, particularly in settings of complex clinical presentations. Table 24–5 summarizes the most frequently used evidence-based test procedures for diagnostic evaluations of ED (Rosen et al, 2004c).
Table 24–5 Evidence-Based Tests for Organic Erectile Dysfunction and Recommendations
| TEST | RECOMMENDATION* |
|---|---|
| Vascular | |
| Dynamic infusion cavernosometry and cavernosography (DICC) | B |
| Intracavernous injection pharmacotesting (ICI) | B |
| ICI and color duplex ultrasound | B |
| Arteriography | C |
| Computed tomography angiography | D |
| Magnetic resonance imaging (MRI) | D |
| Infrared spectrophotometry | D |
| Radioisotope penography | D |
| Audiovisual Sexual Stimulation (AVSS) | |
| Independent or jointly with vascular testing | C |
| With or without: pharmacologic stimulation (oral, ICI) | C |
| Neurophysiologic | |
| Nocturnal penile tumescence and rigidity (NPTR) | B |
| Erectiometer/rigidometer | D |
| Biothesiometry (vibratory thresholds) | C |
| Dorsal nerve conduction velocity | C |
| Bulbocavernosus reflex latency | B |
| Plethysmography/electrobioimpedance | D |
| Corpus cavernosum electromyography (CC-EMG) | C |
| MRI or positron emission tomography scanning of brain (during AVSS) | D |
Modified from Rosen RC, Hatzichristou D, Broderick G, et al. Clinical evaluation and symptom scales: sexual dysfunction assessment in men. In: Lue TF, Basson R, Rosen F, et al, editors. Sexual medicine: sexual dysfunctions in men and women. Paris: Health Publications; 2004. p. 173–220; and Harbour R, Miller J. A new system for grading recommendations in evidence-based guidelines. BMJ 2001;323:334–6.
Vascular Evaluation
Combined Intracavernous Injection and Stimulation
The CIS test serves as a first-line evaluation of penile blood flow because of its basic manner of administration and assessment. The test involves the intracavernous injection of a vasodilatory drug or drugs as a direct pharmacologic stimulus, combined with genital or audiovisual sexual stimulation, and the erectile response is observed and rated by an independent assessor (Donatucci and Lue, 1992; Katlowitz et al, 1993). The test is designed to bypass neurologic and hormonal influences involved in the erectile response and allows the clinician to evaluate the vascular status of the penis directly and objectively.
A normal CIS test, based on the assessment of a sustainably rigid erection, is understood to signify normal erectile hemodynamics. Alternative diagnoses of psychogenic, neurogenic, or endocrinogenic ED may then be considered. However, it is known that false-positive results may occur in up to 20% of patients with borderline arterial inflow (as defined by the measurement of 25 to 35 cm/sec peak cavernous artery systolic flow on duplex ultrasound) (Pescatori et al, 1994). False-negative results are also possible and occur most commonly because of patient anxiety, needle phobia, or inadequate dosage.
Duplex Ultrasonography (Gray Scale or Color Coded)
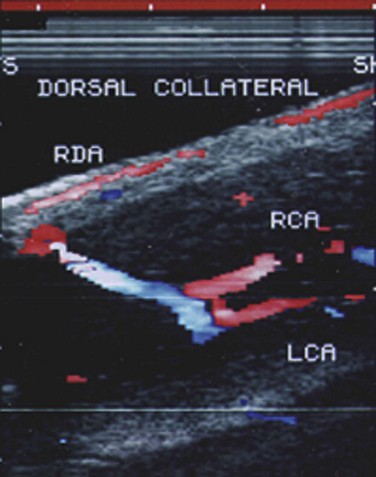
The technique consists of high-resolution (7 to 10 MHz) real-time ultrasonography and color pulsed Doppler, which serves to visualize the dorsal and cavernous arteries selectively and to perform hemodynamic blood flow analysis (Lue et al, 1989). Scanning is applied to the surface of the penis and may include the entire penis from the crura in the perineum to the tip. Color-coded duplex ultrasound indicates the direction of blood flow within vessels, with red designating direction toward the probe and blue designating direction away from the probe (Broderick and Arger, 1993; Herbener et al, 1994). Flow velocities are measured at baseline before injection and commonly every 5 minutes afterwards up to 20 minutes. Cavernous arterial diameters may also be measured. Vascular anatomic communications between the paired cavernous arteries or between the dorsal and cavernous arteries should be noted (Fig. 24–4). Erection quality should also be simultaneously assessed and rated. An observed poor erection, possibly associated with patient anxiety, should prompt vasodilator redosing as recommended for the CIS test.
A standard pattern of Doppler waveforms occurs with hemodynamic changes in corporeal pressure during progression to normal full erection (Fig. 24–5) (Schwartz et al, 1991). In the filling phase when sinusoidal resistance is low (within 5 minutes after vasodilator injection), the waveform increases in size consistent with high forward flow during both systole and diastole. As intracavernous pressure increases, diastolic velocities decrease. With full erection, the systolic waveforms sharply peak and may be slightly less than during full tumescence. At maximal rigidity, when intracavernous pressure exceeds systemic diastolic blood pressure, diastolic flow may be zero. The sonographic color pattern of the cavernous artery may demonstrate an impressive shift from red to blue in association with the reversal of diastolic flow.
Normative values have been described for peak systolic velocity (PSV) and diameter of the cavernous arteries during increases in arterial inflow to the penis. Early studies documented that the PSV of the cavernous arteries consistently exceeded 25 cm/sec within 5 minutes of vasodilator injection in patients with nonarteriogenic causes of ED (i.e., psychogenic, neurogenic) (Lue et al, 1985; Mueller and Lue, 1988). Investigators subsequently confirmed mean PSV of cavernous arteries after pharmacostimulation to range from 35 cm/sec to 47 cm/sec in normal subjects (Benson and Vickers, 1989; Shabsigh et al, 1990). A cut point at 25 cm/sec had a sensitivity of 100% and a specificity of 95% in patients with abnormal pudendal arteriography (Quam et al, 1989). Diameter changes of the cavernous artery after vasodilator injection were found to increase less than 75% and rarely exceed 0.7 mm in patients with severe vascular ED (Lue and Tanagho, 1987; Mueller and Lue, 1988). Importantly, unlike PSV changes, percentage of cavernous arterial vasodilation was not found to correlate well with findings on pudendal arteriography (Jarow et al, 1993).
Vascular arterial anatomic variants may confound the interpretation of duplex ultrasonography (Breza et al, 1989; Jarow et al, 1993). Early cavernous arterial branching or the presence of multiple such branches may affect blood flow velocity determinations of the main cavernous artery. The presence of distal arterial perforators extending from the dorsal or spongiosal arteries also may alter the measurement of cavernous arterial blood flow velocity. Accordingly, the clinician must recognize these variants to avoid making the incorrect diagnosis of arteriogenic ED. On the other hand, asymmetric blood flow of the cavernous arteries may have diagnostic significance. The findings of dissimilar cavernous artery velocity measurements, which are greater than 10 cm/sec between sides, or reversal of flow across a collateral may suggest a significant atherosclerotic lesion (Benson et al, 1993).
Duplex ultrasound measurements are informative for diagnosing vasculogenic ED (Rosen et al, 2004c). Cavernous arterial insufficiency is suggested when PSV is less than 25 cm/sec; a PSV consistently greater than 35 cm/sec defines normal cavernous arterial inflow. Cavernous artery acceleration time (i.e., PSV divided by systolic rise time) greater than 122 msec may also indicate this diagnosis. Cavernous veno-occlusive dysfunction, which refers to failure of erection maintenance despite adequate cavernous arterial inflow, is suggested by assorted sonographic parameters. Generally meaningful at 15 to 20 minutes after stimulatory onset, these parameters include persistent high systolic flow velocities (i.e., PSV > 25 cm/sec) and high end-diastolic flow velocities (EDV > 5 cm/sec), accompanied by rapid detumescence, following stimulatory onset. In addition, vascular resistive index (RI), based on the formula: RI equals PSV minus EDV that is then divided by PSV, has had tremendous diagnostic utility in this regard. The parameter is based on the concept that, as penile intracavernous pressure during erection achievement equals or exceeds diastolic pressure, diastolic flow in the corporal bodies will approach zero and the value for RI will approach one. An RI greater than 0.9 has been associated with normal penile vascular function, and that less than 0.75 is consistent with veno-occlusive dysfunction (Naroda et al, 1996).
Several technical modifications of sonographic evaluation of the penis have been described. A portable Midus pulsed Doppler unit connected to a laptop computer for in-office testing reliably records the Doppler waveform of the cavernous arteries despite not providing a real-time ultrasound image (Metro and Broderick, 1999). Power Doppler offers an even more specialized technique to visualize distal ramifications of the main cavernous artery down to the level of arterioles (Sarteschi et al, 1998; Golubinski and Sikorski, 2002). A somewhat more invasive approach that evaluates the integrity of cavernosal arterial flow involves the measurement of the cavernous artery systolic occlusion pressure (CASOP) by a Doppler transducer during saline intracavernous infusion (Rhee et al, 1995). As a variation on the stimulatory component of penile sonographic testing, a combination of an oral PDE5 inhibitor in association with visual erotic stimulation has been shown to be an effective, noninvasive method (Bacar et al, 2001; Speel et al, 2001). Sonographically measured postocclusive vasodilation of the cavernous arteries, which is believed to relate to the level of intact endothelial function in the penis, has been found to be diagnostic for organic ED (Virag et al, 2004). Cavernous artery intima media thickness as demonstrated by high-resolution echo color Doppler ultrasound has been suggested to be more accurate than PSV in predicting vasculogenic ED (Caretta et al, 2009).
Dynamic Infusion Cavernosometry and Cavernosography
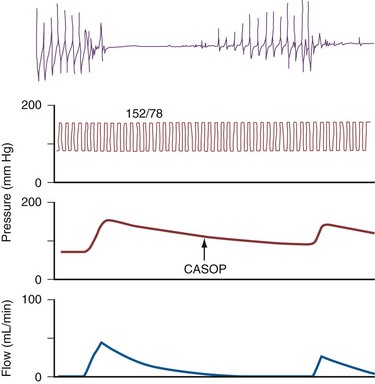
The technique involves two needles inserted into the penis for simultaneous saline infusion and intracavernous pressure monitoring following intracavernous pharmacologic injection. The testing requires complete trabecular smooth muscle relaxation to avoid erroneous results, and repeated and maximal pharmacologic dosing protocols are recommended (Hatzichristou et al, 1995). Measurements of maintenance flow rate, pressure drop, and CASOP are done to verify complete smooth muscle relaxation (Fig. 24–6).
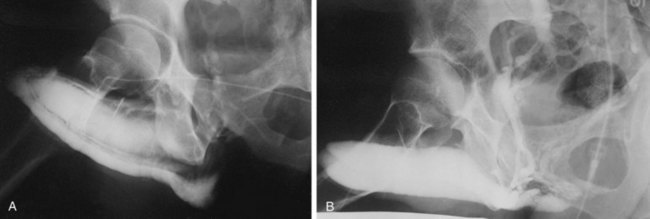
Dynamic infusion cavernosometry and cavernosography evaluate the penile venous outflow system. The existence of veno-occlusive dysfunction is indicated by the failure to increase intracavernous pressure to the level of the mean systolic blood pressure with saline infusion or the demonstration of a rapid drop of intracavernous pressure after cessation of saline infusion (Puyau and Lewis, 1983; Rudnick et al, 1991; Shabsigh et al, 1991; Motiwala, 1993). The flow rate required to maintain erection at an intracavernous pressure of more than 100 mm Hg is normally less than 3 to 5 mL/min, and the pressure decrease in 30 seconds from 150 mm Hg is normally less than 45 mm Hg. Cavernosography follows cavernosometric evaluation and is intended to reveal the site of venous leakage (Fig. 24–7). With normal veno-occlusive function, there should be opacification of the corpora cavernosa with minimal or no visualization of venous structures or corpus spongiosum. With impaired veno-occlusive function, leakage may be identified into such sites as the glans, corpus spongiosum, superficial dorsal veins, and cavernous and crural veins. More than one site is visualized in the majority of patients (Lue et al, 1986; Rajfer et al, 1988; Shabsigh et al, 1991).
Penile Angiography
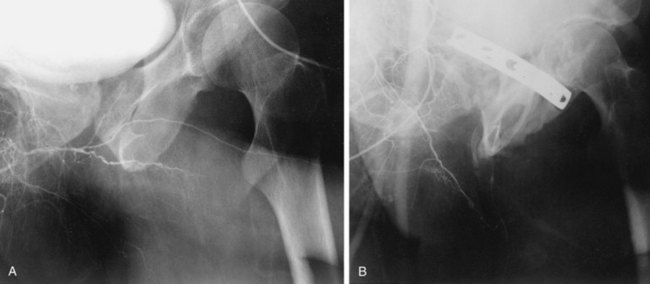
The procedure involves selective cannulation of the internal pudendal artery and injection of radiographic contrast. The intracavernous injection of a vasodilating agent is optimally used to induce maximal vasodilation of the penile arterial supply. The anatomy and radiographic appearance of the iliac, internal pudendal, and penile arteries are then evaluated and documented (Fig. 24–8). The inferior epigastric arteries are frequently studied as well to determine their suitability for use in surgical revascularization. It should be recognized that significant variation of the intrapenile arterial anatomy exists, challenging the angiographer to differentiate congenital variations from acquired abnormalities and establish their clinicopathologic relevance (Bähren et al, 1988; Benson et al, 1993).
Historical and Investigational Studies of Penile Blood Flow
Penile Brachial Pressure Index
This test refers to the penile systolic blood pressure divided by the brachial systolic blood pressure. The technique involves applying a small pediatric blood pressure cuff to the base of the flaccid penis and measuring the systolic blood pressure with a continuous-wave Doppler probe. A PBI of 0.7 or less has been used to indicate arteriogenic ED (Metz and Bengtsson, 1981). The technique has not been found to be valid, basically because it does not assess the hemodynamic properties of a functionally relevant, induced erection, and thus it is not recommended for use (Aitchison et al, 1990; Mueller et al, 1990).
Penile Plethysmography (Penile Pulse Volume Recording)
This test evaluates arterial pressure waveforms in the penis with an aggregate of the contributions of all penile vessels (Kedia, 1983). It requires the application of a 2.5- or 3-cm cuff connected to an air plethysmograph applied to the base of the penis, inflating the cuff to a pressure above brachial systolic pressure, and then decreasing the pressure by 10–mm Hg increments while recording pressure waveform tracings. Abnormal pressure waveforms by diagnostic criteria have been used to indicate vasculogenic ED (Doyle and Yu, 1986). Because this study is done in the flaccid penis like the PBI, its clinical relevance has been questioned. Despite this concern, a technical modification that measures postischemic flow-mediated dilation was introduced as being informative regarding penile vascular endothelial function (Dayan et al, 2005; Vardi et al, 2009).
Radioisotopic Penography
This test quantifies changes in penile blood volume after intracavernous injection of a vasoactive agent using 99mTc-labeled red blood cells (Shirai et al, 1976). Extremely low flow is understood to mean arteriogenic ED (Smith et al, 1998). An evaluation comparing color Duplex ultrasound and radionuclide penography showed poor correlation (Glass et al, 1996).
Penile Magnetic Resonance Imaging
This test has significant potential applications for the assessment of anatomic details of the penis and penile microcirculation. Angiographic techniques may be combined with it to evaluate the anatomic condition of the internal iliac and penile vasculature. Magnetic resonance angiography has been shown to have good correlation with color duplex ultrasound testing (Stehling et al, 1997; John et al, 1999).
Penile Near-Infrared Spectrophotometry
This test provides continuous, quantitative measurements of penile blood flow using a specialized near-infrared spectrophotometry instrument (Burnett et al, 2000). It may be applied with an erectile stimulus and documents the hemodynamic phenomena of erection. Penile spectrophotometry has been further investigated in combination with intraurethral pharmacotherapy documenting blood flow increase to the penis with this erectogenic modality (Padmanabhan and McCullough, 2007). Further investigation of this technique is necessary to establish its clinical utility.
Cavernous Smooth Muscle Content
This test evaluates the smooth muscle composition of the corporeal tissue by light microscopic and computed morphometric assessment of biopsies of the penis and may serve adjunctively in the diagnosis of vasculogenic ED (Wespes et al, 1992). A reduced proportion of corporeal smooth muscle (and correspondingly increased collagen) has been observed in older men with veno-occlusive dysfunction (19% to 36% smooth muscle) and arteriogenic ED (10% to 25%), compared with that of young, healthy men with normal erections and penile curvature (40% to 52%) (Wespes et al, 1991). In part because of its invasiveness, the test is controversial and thus it remains investigational at present.
Psychophysiologic Evaluation
Penile Tumescence and Rigidity Monitoring
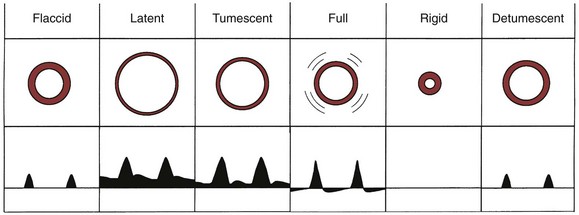
Nocturnal penile tumescence (NPT) monitoring, which describes the study of erections that occur with nighttime sleep, was classically described as a technique offering assessment of physiologic erectile ability (Wasserman et al, 1980). Standardly, sleep laboratory nocturnal penile tumescence and rigidity (NPTR) testing applies nocturnal monitoring devices that measure the number of episodes, tumescence (circumference change by strain gauges), maximal penile rigidity, and duration of nocturnal erections (Kessler, 1988). The conventional approach is to perform monitoring in conjunction with electroencephalography, electro-oculography, and electromyography (EMG), with nasal airflow and with oxygen saturation to document rapid eye movement (REM) sleep and the presence or absence of hypoxia (sleep apnea). Importantly, documentation of REM sleep is done because of the observation that true erectile phenomena occurring during sleep are associated with the REM sleep phase (Fisher et al, 1965). Sleep movement patterns are also monitored because periodic limb movement disorders are associated with abnormal NPT. Axial rigidity is measured along with photography of the erect penis on awakening the patient at maximal tumescence; a buckling device is applied to the tip of the penis to measure resistance (500 g minimum for vaginal penetration, 1.5 kg suggestive of complete rigidity) (Karacan et al, 1977). NPT has traditionally been performed over two to three nights to overcome the so-called first-night effect when REM sleep is inconsistent. Formal testing, which involves a specially equipped sleep laboratory staffed with trained observers, is costly. The monitoring of diurnal penile tumescence, in reference to monitoring performed during daytime napping, has served alternatively as an in-office evaluation (Morales et al, 1994).
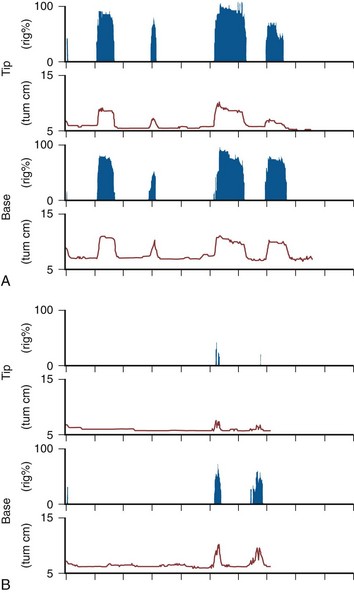
Rigiscan (Timm Medical Technologies, Inc. Minneapolis, MN) is an automated, portable device used for NPTR, which combines the monitoring of radial rigidity, tumescence, number, and duration of erectile events (Bradley et al, 1985). The device employs two loops, one placed at the base of the penis and the other placed at the coronal sulcus (respectively, base and tip recording sites), which record penile tumescence (circumference) and radial rigidity with timed, standardized constrictions of the loops. A baseline initialization is done with the patient in the office, and then it is calibrated for home use. At home, registrations of penile rigidity are done every 3 minutes and increased to every 30 seconds when the base loop detects a circumference increase of greater than 10 mm (Fig. 24–9). Recommended criteria for normal NPTR include four to five erectile episodes per night, mean duration longer than 30 minutes, an increase in circumference of more than 3 cm at the base and more than 2 cm at the tip, and maximal rigidity above 70% at both base and tip (Cilurzo et al, 1992). A computerized program has yielded standardized data measurements according to cumulative distribution of time-intensity measures, defined as tumescence activity units (TAU) and radial rigidity activity units (RAU) (Burris et al, 1989; Levine and Carroll, 1994). Potential limitations of Rigiscan include that radial rigidity does not accurately predict axial rigidity (Allen et al, 1993; Licht et al, 1995) and considerable variability apparently exists even in normal subjects (Levine and Carroll, 1994). Further, the manner of testing does not allow verification of the presence of REM sleep.
NPT electrobioimpedance (NEVA, American Medical Systems, Inc., Minnetonka, MN) is a more recently introduced device that assesses volumetric changes in the penis during nocturnal erections (Knoll and Abrams, 1999). The device consists of three small electrode pads applied to the hip and the penile base and glans and a small recording device attached to the patient’s thigh. In operation, an undetectable alternating current is transmitted from the glans electrode to the hip ground, and the penile base electrode measures impedance and changes in penile length. Impedance measures decrease in concert with increases in cross-sectional area of the penis during nocturnal tumescence. Further investigation is necessary to establish the relationship of volumetric changes and rigidity of the penis. Similar to Rigiscan, the technique does not include REM sleep monitoring and correlations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 -inch needle (27 to 29 gauge), which is inserted at the lateral base of the penis directly into the corpus cavernosum for medication delivery. After needle withdrawal, manual compression is applied to the injection site for 5 minutes to prevent local hematoma formation. The assessment is done periodically afterwards to rate both rigidity and duration of response. Repeated dosing may be performed if the initial erectile response is poor. Return to penile flaccidity is required before allowing the patient to leave the office, and if detumescence does not occur spontaneously in approximately an hour after dosing, intracavernous injection of a diluted phenylephrine solution (500 µg/mL) can be done every 3 to 5 minutes until flaccidity returns.
-inch needle (27 to 29 gauge), which is inserted at the lateral base of the penis directly into the corpus cavernosum for medication delivery. After needle withdrawal, manual compression is applied to the injection site for 5 minutes to prevent local hematoma formation. The assessment is done periodically afterwards to rate both rigidity and duration of response. Repeated dosing may be performed if the initial erectile response is poor. Return to penile flaccidity is required before allowing the patient to leave the office, and if detumescence does not occur spontaneously in approximately an hour after dosing, intracavernous injection of a diluted phenylephrine solution (500 µg/mL) can be done every 3 to 5 minutes until flaccidity returns.