Fig. 39.1
Zonal anatomy of the prostate (Reprinted from McNeal [2]. With permission from Wolters Kluwer Health)
The peripheral zone (PZ) (Fig. 39.1) accounts for 70 % of glandular tissue covering the posterior and lateral parts of the gland accounting for 70 % of cancers. Its ducts drain into the prostatic sinus along the entire length of the prostatic urethra. The anterior fibromuscular stroma is the nonglandular part of the prostate and accounts for up to one-third prostate mass. It is rarely invaded by carcinoma.
Incidence and Epidemiology
Prostate cancer (PCa) is now recognised as one of the most important medical problems facing the male population [3]. For most men PCa is slow growing and does not result in clinical signs or symptoms during their lifetime [4, 5]. Despite its slow growth, in some men PCa progresses and is a leading cause of cancer morbidity and mortality. Globally, it is a less prominent cause of cancer death, contributing 5.8 % of cancer deaths in men [3]. Since 1985, there has been a slight increase in most countries in the number of deaths from PCa, even in countries or regions where PCa is not common [6]. Despite significant advances in the treatment of PCa, it remains a growing problem for men’s health.
Worldwide, PCa is the second most prevalent cancer and the sixth leading cause of cancer death in males, accounting for 14 % (903,500) of the total new cancer cases and 6 % (258,400) of the total cancer deaths in males in 2008 [7]. In the United States (US), PCa is the most commonly diagnosed visceral cancer; in 2011, there were expected to be 241,000 new PCa diagnosis and about 34,000 PCa deaths. PCa is second only to nonmelanoma skin cancer and lung cancer as the leading cause of cancer and cancer death, respectively, in US men [8]. Currently, around three million European men are living with PCa and this number will grow due to population aging. There were 350,000 new cases of prostate cancer diagnosed in the EU27 (27 European countries) in 2008, which accounts for about 70 new cases per 100,000 men across the EU27 each year. However, the incidence varies considerably between various European states, ranging from 14 per 100,000 in Turkey to over 123 per 100,000 in Ireland [9].
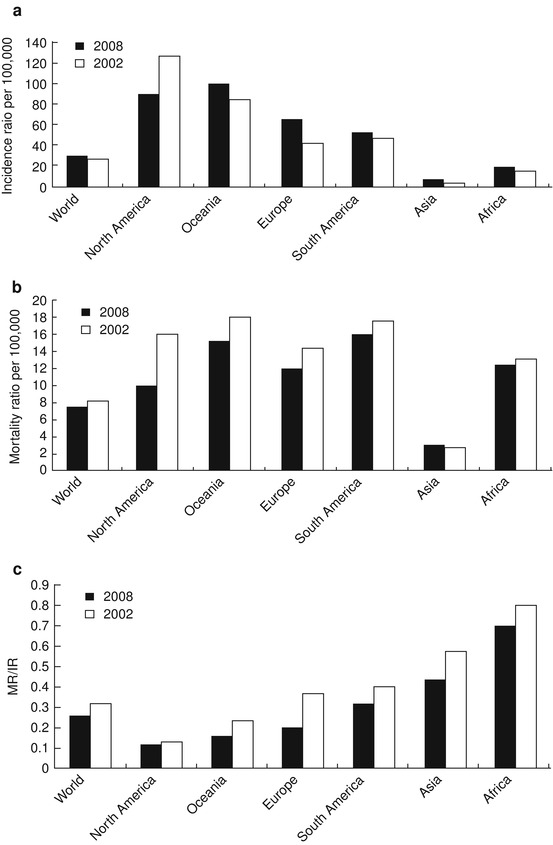
The worldwide PCa mortality-to-incidence rate (MR/IR) ratios from the International Agency for Research on Cancer (IARC) have shown a marked decline from 2002 to 2008 [10, 11]. But new PCa cases worldwide have increased from 679,060 to 914,000 during this period. As shown in Fig. 39.2, PCa incidence rates increased from 25.3/105 to 28.5/105 person year (PY), while mortality rate decreased from 8.2/105 to 7.5/105 PY. The MR/IR ratio has decreased from 0.32 to 0.26. The PCa incidence rate in North America has decreased from 119.9/105 to 85.7/105 PY, and the mortality rate has decreased from 15.8/105 to 9.9/105 PY, accompanied with a decrease in the MR/IR ratio from 0.13 to 0.12. Of all the continents, Europe had the most remarkable change. The PCa incidence rate increased from 40.0/105 PY in 2002 to 61.4/105 PY in 2008. However, the mortality rate decreased from 14.2/105 to 12.1/105 PY and the MR/IR ratio decreased from 0.36 to 0.20. This remarkable progress could be attributed to the European Randomized Study on Screening for PCa [12]. Although Asia and Africa have shown some improvements in mortality rates and MR/IR ratios, the changes are modest compared to other continents.
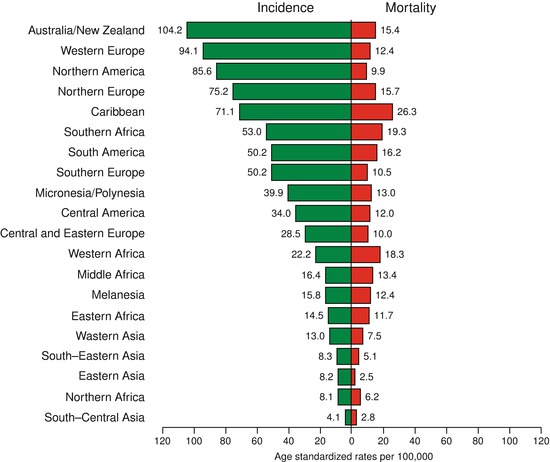
PCa affects elderly men more often and therefore is a bigger health concern in developed countries. There are large regional differences in incidence rates of PCa. The highest incidence rates are in the developed countries, with the lowest in Africa and Asia. About 15 % of male cancers are PCa in developed countries compared with 4 % of male cancers in developing countries [11]. Incidence rates vary by more than 25-fold worldwide, with the highest rates recorded primarily in the developed countries of Oceania (Australia and islands around it), Europe, and North America (Fig. 39.3), largely because of the wide utilization of PSA testing that detects clinically important tumors as well as other slow-growing cancers that might otherwise escape diagnosis.
In contrast, men of African descent in the Caribbean region have the highest PCa mortality rates in the world, which is thought to reflect partly a difference in genetic susceptibility [13, 14, 15].
The majority of PCa cases are slow growing and do not pose an immediate threat to the individual. The lifetime risk of a man of 50 years of age having microscopic evidence of PCa is 42 %, while his risk of dying from the disease is only 3 % [16]. Many more cases of PCa do not become clinically evident, as indicated in autopsy series, where PCa is detected in one-third of men under the age of 80 and in two-thirds of older men [17]. These data suggest that PCa often grows so slowly that most men die of other causes before the disease becomes clinically advanced. However, there is a type of prostate cancer that can occur in younger and older men which is more aggressive and leads to a more rapid death if not detected early enough.
The most complete information available on PCa epidemiology in the US is provided by the Surveillance Epidemiology and End Results (SEER) program of the US National Cancer Institute [18]. There was a gradual rise in the pre-PSA era that could be explained by the rising rates of PCa diagnosed through the transurethral resection of prostate. As the PSA era began, an abrupt rise in PCa incidence was observed, peaking in 1992 (237 per 100,000 person-years). A subsequent decline in the incidence followed until 1995 and was attributed to the cull effect, whereby removal of detectable cases in prior years resulted in fewer available cases for repeat screening. A relatively stable incidence was observed in 1995–1999, but the rates were higher than that prior to the PSA era. Rates have since fluctuated, falling to 169 in 1995, rising again to 184 in 2001, and most recently falling again, showing rates of 171 and 153 for 2007 and 2008, respectively [19] (Fig. 39.4).
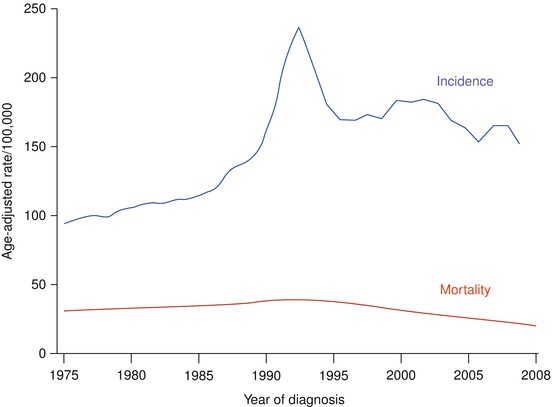

Fig. 39.4
PCa: Changes over time average annual age-adjusted incidence and mortality rates in the United States, 1975–2008 (Based on data from SEER Program)
Before the PSA screening era, PCa was detected either by symptoms, or incidentally on final pathology after transurethral resection or open simple prostatectomy for presumed benign prostatic hyperplasia [20]. Thus, presentation with advanced and metastatic disease was more common in the pre-PSA era [21]. At the start of the PSA screening era, a large spike in the number of PCa diagnoses occurred in the US, with over 1.3 million additional diagnoses of PCa by 2005 compared to rates before 1986 [22]. A similar increased incidence of PCa did not occur in countries and regions where PSA-based screening was less common [23].
Related to the increased incidence of PCa detection, the proportion of men with low-stage PCa increased dramatically in the US at the start of the screening era [24]. When incidence rates are examined by grade, most of the increase in the PSA era was attributable to moderately differentiated tumors, accounting for approximately 80 % of the increase in incidence. Moderately differentiated tumors became the predominant grade of prostate cancer in 1984. A rapid increase in moderate differentiation was observed after 1988. Seventy-six percent of the increase in overall incidence is accounted for by moderately differentiated tumors and 8 % by well-differentiated tumors. Poorly differentiated tumors were more common than well-differentiated tumors after 1991.
Screening
Screening has been advocated as a means of detecting PCa in the early stages, which are amenable to local interventions with curative intent, to decrease overall and disease specific mortality [25]. The long latent period of PCa and its potential curability make this disease an excellent candidate for screening strategies that attempt to find disease in an early curative state.
The ideal screening test is minimally invasive, easily available and performed, acceptable to the general population, accurate, and significantly affects such outcomes of the disease as the mortality rates [26]. In screening, success depends on the capacity of a test to find disease that requires cure, and on its ability to improve disease-specific mortality [27]. While the intention of screening for PCa is to decrease mortality and increase health-related quality of life (HRQL), the true benefit of screening for PCa remains uncertain [28].
Screening for PCa to date has been performed with digital rectal examination (DRE) and PSA testing. Use of the DRE as a screening tool is limited due to poor reliability, sensitivity, and the inability to palpate the entire prostate gland, especially for small tumours that have not reached the prostatic capsule [29]. Most PCas develop in the peripheral zone of the prostate, making them palpable on DRE when sufficiently large. However, palpable cancers are often already advanced in both grade and stage, and are potentially no longer organ confined.
PSA elevations can arise without a palpable abnormality, the opportunity exists to use PSA to find PCa in an early, localized stage. This ability to find early PCas made PSA an interesting biomarker to use for screening for PCa [30, 31]. Despite the ability of PSA screening to find early stage PCa, controversy exists over its ability to save lives. Autopsy studies have shown that even men without a clinical diagnosis of PCa have a large reservoir of asymptomatic disease. Many men die with, but not of, PCa. A screening test such as the PSA test that cannot differentiate between lethal and non lethal disease allows men with potentially lethal PCa to be diagnosed and treated in the early stages of their disease. However, men with nonlethal disease do not benefit from their diagnosis and subsequent treatment. Such men, in whom PCa was never destined to cause clinical symptoms, are “overdiagnosed”, and if they receive treatment, they are “overtreated”. Overdiagnosed patients carry the burden of a cancer diagnosis that they would not have received in the absence of screening; overtreated patients suffer the short-term and long-term adverse effects of treatment they did not need. With any screening test there is some element of both of these phenomena; for PSA testing, the low specificity of the test and its inability to distinguish latent from aggressive cancer means that a substantial number of men are overdiagnosed with and overtreated for PCa [27, 32]. Recent data has suggested that the PSA test does not attain the likelihood ratios (i.e. the likelihood of a given test result in a person with the disease compared with the likelihood that the same result would be apparent in a person without the disease) for a screening test, regardless of what threshold value for the PSA is assigned [33].
Population based recommendations for cancer screening should ideally be based on high quality evidence derived from systematic reviews of randomised controlled trials (RCTs) that document a positive impact of screening on outcomes that are the most important to patients [34]. In 2006, a systematic review published in the Cochrane Library concluded that there was insufficient evidence to either support or refute the routine use of mass, selective, or opportunistic screening compared with no screening [28]. This Cochrane systematic review was based on two randomised controlled trials that enrolled 55,512 participants overall but was limited by substantial methodological weaknesses in the design, conduct, and analysis of the included studies. The evidence drawn from this systematic review did not show that screening improved outcomes. By 2010, four additional trials [12, 35–37] enrolling 351,531 participants had been published, thereby providing strong impetus for an updated synthesis of research evidence.
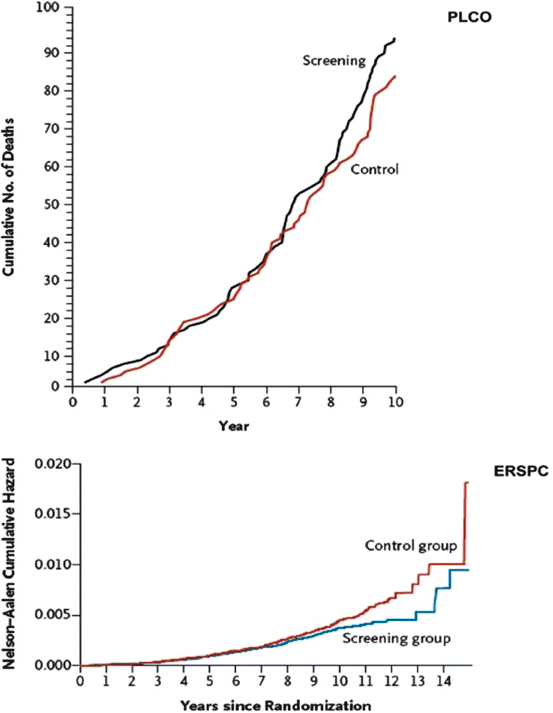
To evaluate the efficacy of PCa screening, two high profile RCTs of PCa screening were published: the Prostate, Lung, Colorectal, and Ovary (PLCO) trial in the United States [36] and the European Randomised Study of Screening for Prostate Cancer (ERSPC) in Europe [12]. They have significantly contributed to our current knowledge and understanding of PCa screening, as well as the difficulties and controversies associated with their ambiguous findings. The ERSPC trial used data from seven centers in different European countries, with a total of 167,387 men undergoing randomization [12, 38]. Slightly different methods and follow-up routines were used in the different countries. PSA cut-off varied from 3 to 4 ng/ml among countries, with rates necessitating further testing ranging from 2.5 to 3.9 ng/ml. With average and median follow-up times of 8.8 and 9.0 years, respectively, there were 214 PCa related deaths in the screening group and 326 in the control group. These data result in an unadjusted rate ratio for death in the screening group of 0.80 (95 % confidence interval [CI], 0.67–0.95; p = 0.01) and an adjusted rate ratio of 0.80 (95 % CI, 0.65–0.98; p = 0.04). In other words, to prevent one death from PCa, 1,410 men (95 % CI, 1,132–1,721) would need to be screened and 48 would need to be treated. The ERSPC investigators concluded that PSA based screening reduced the rate of death from PCa by 20 % but was associated with a high risk of over treatment.
In the PLCO trial, 76,639 men at ten US study centers were included in the trial [36]. The incidence of death per 10,000 person-years was 2.0 (50 deaths) in the screening group and 1.7 (44 deaths) in the control group (rate ratio: 1.13; 95 % CI, 0.75–1.70). The authors concluded that after an average 7-year of follow-up, mortality did not significantly differ between the two groups, and therefore, in this study, screening was not associated with mortality (rate ratio: 1.13) [38].
The curves representing the cumulative risk of death from PCa in the ERSPC in comparison with the PLCO study is shown in Fig. 39.5. Visual comparison allows judgment of the differences seen in the two studies. Clearly, the PLCO study does not reach the goal of the original power calculation of identifying a 20 % difference in PCa mortality between screening and control. Reasons for the difference between PLCO and ERSPC are evident from the two publications. ERSPC recruited more than two times as many participants. The average follow-up is 2 years shorter in the PLCO study. A PSA cut-off of 4 was used, and compliance with biopsy indications was low as revealed in [36, 39]. Prior PSA testing occurred in 44 % of men prior to randomization into the study. This has led to a decrease in the number of cancers found in screening. In addition, 53 % of the men in the control group underwent screening. This, considering the 85 % compliance with the PSA testing in the screening arm, leaves only a window of 33 % between the two study arms. On the basis of these characteristics, it is unlikely that the PLCO study will ever contribute to determine the value of screening in lowering PCa mortality [40].
There is currently no evidence for introducing widespread population-based screening programmes for early PCa detection in all men [41]. Based on the results of these two large randomised trials, most if not all of the major urologic societies have concluded that at present widespread mass screening for PCa is not appropriate. Rather, early detection (opportunistic screening) should be offered to the well-informed man. Few organizations support routine PSA screening. The American Urological Association advocates routine PSA screening [42], and its current guidelines, updated after the publication of the PLCO and ERSPC trials, recommend a baseline PSA measurement for men when they reach 40 years of age. This measurement should be followed by tailored surveillance and biopsy based on multiple clinical factors, and no set PSA cut point applies for all patients [42]. By contrast, both the European Association of Urology [43] and the Japanese Urologic Association [44] offer no recommendation for routine PSA screening. The European Association of Urology (EAU) specifically states that its lack of recommendation is due to the large risk of overtreatment of PCa after PSA screening.
National cancer prevention organizations offer at most limited support for PSA screening. The American Cancer Society offers no recommendation for routine testing for PCa with PSA [45] (13.31). They instead advise that patients should be offered the opportunity to make an informed decision about PSA screening based on the potential risks and benefits of the test. The American College of Preventive Medicine also offers no recommendation for screening for PCa [46] (13.32). In the UK, the National Health Service (NHS) has no organized screening programme [47]. Finally, the US Preventative Services Task Force specifically recommends against PSA screening. Their guidelines state that screening should not be performed in men older than 75 years, and that the benefits of screening in younger men are uncertain [25].
Given the important tradeoffs between potential benefits and harm involved with either screening or not screening for PCa, and the lack of definitive data on screening outcomes, it is particularly important that patients make informed decisions about undergoing testing. The United States Preventive Services Task Force Guidelines [48], American College of Physicians [49], American Urologic Association [50], American Cancer Society [51], and the Canadian Task Force on the Periodic Health Examination [52] all stress the importance of informed decision making.
The American College of Physicians and the American Cancer Society have provided useful summaries of discussion points to consider when counseling patients about PCa screening [49, 51]:
PCa is an important health problem; it is one of the most frequently diagnosed cancers in the United States and a leading cause of cancer death in men.
Men should be involved in making the decision whether or not to be screened.
PCa screening may reduce the chance of dying from PCa. However, the evidence is mixed and the absolute benefit is small.
PCa screening is associated with a substantial risk of being diagnosed with PCa. Many cancers detected by screening are considered “overdiagnosed”, meaning that they never would have caused problems during a man’s lifetime.
In order to determine whether a cancer is causing an abnormal test, men need to undergo a prostate biopsy. However, the PSA test and digital rectal examination (DRE) can both have false positive and false negative results and prostate biopsies may also miss finding cancers.
The PSA blood test with or without the DRE can detect cancer at an earlier stage than when cancers are found because they are causing problems.
Aggressive therapy is necessary to realize any benefit from finding an early-stage PCa.
Surgery and radiation therapies are the treatments most commonly offered in an attempt to cure PCa; however, they can lead to problems with urinary, bowel, and sexual function.
No current tests can accurately determine which men with a cancer found by screening are most likely to benefit from aggressive treatment (i.e., those whose cancers are destined to cause health problems). Most men with PCa will die from other causes, many will never experience health problems from their cancer.
A strategy of active surveillance may be appropriate for men who are at low risk for complications from PCa. This means not immediately treating a cancer but following PSA tests, DRE, and repeating biopsies to determine whether aggressive treatment is indicated because the cancer is progressing.
Clinicians find it challenging to provide comprehensive, consistent, and balanced information about PCa screening decisions during clinic visits [53]. Consequently, efforts have focused on using decision aids to help patients understand screening issues and make informed decisions for screening [54].
Pathology
Adenocarcinoma accounts for the vast majority of malignant growths of prostate. The location they arise from corresponds to McNeal’s zones [2]: peripheral (70 %), transitional (20 %), and central (1–5 %). It is multifocal in more than 85 % of cases. The Union Internationale Contre le Cancer (UICC) 2009 TNM classification is used throughout the guidelines [55] (Table 39.1). TNM Classification is also used for the diagnosis and management of prostate cancer.




Table 39.1
TNM classification of prostate cancer
T – Primary tumour | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
T1 | Clinically unapparent tumor not palpable or visible by imaging |
T1a | Tumor incidental histological finding in 5 % or less of tissue resected |
T1b | Tumor incidental histological finding in more than 5 % of tissue resected |
T1c | Tumor identified by needle biopsy (e.g., because of elevated PSA level) |
T2 | T2 Tumor confined within the prostate |
T2a | Tumor involves one half of one lobe or less |
T2b | Tumor involves more than half of one lobe, but not both lobes. |
T2c | Tumor involves both lobes |
T3 | Tumor extends through the prostatic capsule |
T3a | Extracapsular extension (unilateral or bilateral) including microscopic bladder neck involvement |
T3b | Tumor invades seminal vesicle(s) |
T4 | Tumor is fixed or invades adjacent structures other than seminal vesicles: external sphincter, rectum, levator muscles, and/or pelvic wall |
N- Regional lymph nodes | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
M- distant metastasis | |
MX | Distant metastasis cannot be assessed |
M0 | No distant metastasis |
M1 | Distant metastasis |
M1a | Non-regional lymph node(s) |
M1b | Bone(s) |
M1c | Other site(s) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree