Environmental and Lifestyle Issues in Colorectal Cancer
Elizabeth T. Jacobs
Patricia A. Thompson
María Elena Martínez
Introduction
Worldwide, colorectal cancer accounted for 1 million new cases and 500,000 deaths in 2002 (1). Rates of this malignancy vary by country. Although cancer of the colorectum is rare in developing countries, this malignancy is the second most frequently diagnosed in developed countries. Wide geographic variation in colorectal cancer incidence and mortality rates is believed to be due to lifestyle/environmental factors. Migrant studies, which compare individuals who move from countries with low rates to those with high rates, also suggest that lifestyle/environmental factors influence the development of colorectal cancer. Furthermore, incidence rates of this disease are increasing in some countries with formerly low rates, which also suggest that lifestyle/environmental factors are involved in their etiology.
The presentation that follows begins with a review of the descriptive epidemiology, followed by a summary of results of analytic epidemiologic studies. Prior to the discussion of the published work, a review of study designs is provided to familiarize the reader with these concepts. The summary further focuses on epidemiologic studies pertaining to obesity, physical activity, hormone replacement therapy (HRT), tobacco, nonsteroidal antiinflammatory drugs (NSAIDs), and diet.
Descriptive Epidemiology
Incidence and Mortality Rates Worldwide
In general, the incidence of colorectal cancer is rising worldwide, although there are a few exceptions. However, mortality rates are not rising as rapidly as incidence rates and have dropped significantly in Canada, the United States, and some European countries, which is possibly the result of improved survival.
Countries in Asia and Oceania show a 10-fold range in variation in colorectal cancer incidence. Among these countries, the largest rise in incidence has been seen in Japan, where incidence increased at a rate of 20% to 30% per 5-year period from 1970 to 1985 (2). Of interest, rates among Japanese living in Hawaii are also rising. Overall, in most of the countries of Asia and Oceania, there have been increases among Japanese and Chinese populations, whereas no significant change or declines were seen among the low-risk populations of India. As well, incidence rates in Australia have been rising by 12% to 14% every 5 years, although no such rise in mortality rates has been shown (2).
Fig. 38.1 shows wide international geographic variations in mortality rates for colorectal cancer. Rates of this malignancy have increased by approximately 25% since 1965 in Japan. In the eastern countries of Czechoslovakia, Hungary, Poland, and Yugoslavia, mortality rates have also been increasing steadily. Fig. 38.1 also shows that the male-to-female ratio for colorectal cancer in high-risk areas is higher than that in low-risk areas. Another interesting fact related to international variation in colorectal cancer involves the geographic distribution for cancers of the colon and rectum. In locations considered to be high risk, the ratio of colon to rectal cancer incidence is approximately 2:1 or more, whereas in low-risk regions, the ratio is close to one.
Incidence and Mortality Rates in the Americas and the United States
Incidence rates of colorectal cancer have been rising steadily in areas of Central and South America (2). In the United States, however, decreases in colorectal cancer incidence rates began in the mid-1980s, and have occurred among both males and females. Incidence rates of colorectal cancer decreased by an average of 1.8% per year between 1998 and 2002 (3). Based on 1998 to 2002 data, incidence rates in the United States are 65.9 per 100,000 for colon and 47.9 per 100,000 for rectal cancer.
In 2006, of the estimated 148,610 new colorectal cases that will occur in the United States, 55,170 will die of this disease (3). Colorectal cancer incidence rates increase with age, with 86% of cases occurring in people 55 years and older. In North America, mortality rates from this cancer have been falling significantly. In the United States, annual age-standardized colorectal cancer mortality rates peaked in the 1940s and have steadily fallen since the 1950s (3). The age-standardized mortality rate between 1998 and 2002 was 24.7 per 100,000 for colon and 17.4 per 100,000 for rectal cancer (3).
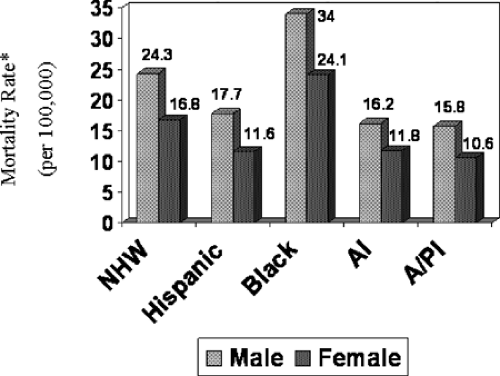
In the United States, differences in colorectal cancer incidence and mortality rates among various racial/ethnic groups are evident (3). Incidence rates of this disease are highest among blacks (72.5/100,000 in males and 56.0/100,000 in females), intermediate among non-Hispanic whites (NHWs) (61.7/100,000 in males and 45.3/100,000 in females), and lower among Hispanics (48.3/100,000 in males and 32.3/100,000 in females) and Native Americans/Alaskan Natives (36.7/100,000 in males and 32.2/100,000 in females). Fig. 38.2 shows that the highest mortality rates are found among blacks. The age-standardized mortality rate for black men from 1998 to 2002 was 34.0 per 100,000; the rate for black women was 24.1 per 100,000. In addition, when compared to other nonwhite populations, black men and women are twice as likely to die of colorectal cancer. These data clearly indicate that the greatest racial/ethnic disparity is shown for mortality rates in
blacks. Although incidence and mortality rates of colorectal cancer are lower in Asian/Pacific Islanders, Hispanics, and Native Americans/Alaskan Natives, it will be important to monitor future trends because there are suggestions in the literature that they are on the rise in some of these racial/ethnic groups. Data from the New Mexico Tumor Registry showed that colon cancer rates increased 3.6% per year among Hispanics between 1969 and 1994 (4). Furthermore, although a decline in incidence rates was shown among Hispanics in California (5), the data show that this decline is not as pronounced as that for NHWs or other racial/ethnic groups.
blacks. Although incidence and mortality rates of colorectal cancer are lower in Asian/Pacific Islanders, Hispanics, and Native Americans/Alaskan Natives, it will be important to monitor future trends because there are suggestions in the literature that they are on the rise in some of these racial/ethnic groups. Data from the New Mexico Tumor Registry showed that colon cancer rates increased 3.6% per year among Hispanics between 1969 and 1994 (4). Furthermore, although a decline in incidence rates was shown among Hispanics in California (5), the data show that this decline is not as pronounced as that for NHWs or other racial/ethnic groups.
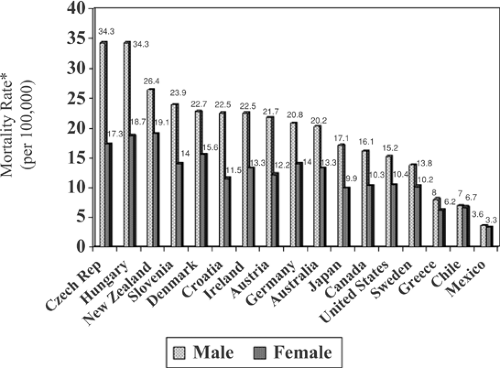 FIGURE 38.1. Mortality rates (per 100,000) of colorectal cancer for selected countries around the world. *Rates are age-adjusted to the World Health Organization world standard population. |
Survival
Between the 1970s and 1990s, 5-year colorectal cancer survival rates in the United States have increased from 50% to 63% in NHWs (6), with current survival rates being at 64% (3). The global picture, however, clearly shows that survival is lower in countries outside the United States: 41% in Europe, 42% in India, and 32% in China (7). Data for survival in the United States also indicate significant racial/ethnic group disparities, where less pronounced increases in survival are observed for certain groups compared to NHWs (8). For example, comparing survival rates from 1975 to 1987 to those in 1988 to 1997, rates among NHW males increased by 10%, and those for black males increased by 9%; however, this change was only 7.4% for Hispanics and 6.4% for Hawaiian natives. For females, changes in survival for the same period of time were 9.2% for NHWs, 6.5% for Hispanics, 5.9% for blacks, and 4.6% for Native Americans/Alaskan Natives, and these actually decreased by 1.3% for Hawaiian natives.
In the United States, additional disparities by racial/ethnic group also present for stage of disease at diagnosis, which clearly affects survival. For example, 16% of colorectal cancer cases are diagnosed with distant disease among NHWs, whereas 20% of Hispanics present with distant disease, which is similar to what is observed for African Americans (9). Furthermore, as noted by Clegg et al. (8), although the proportion of individuals who present with distant disease at diagnosis has decreased among NHW and African Americans, this has actually increased among Hispanics and Native
Americans/Alaskan Natives. These data show that the proportion of Hispanics who presented with distant colorectal cancer increased from 19.8 to 20.3 from 1975 to 1987 to 1988 to 1997; among Native Americans/Alaskan Natives, the increase was more pronounced, from 19.8 to 24.4. More recently published data show similar findings. Using 11 Surveillance, Epidemiology, and End Results cancer registries, Chien et al. (10) showed various racial/ethnic disparities in risk of advanced stage colorectal cancer. One of the strengths of this study is the ability of the investigators to subdivide specific racial/ethnic groups, such as Hispanics, into the various subpopulations. For example, compared to NHWs, men and women of Mexican descent were significantly more likely to present with stage III or IV disease.
Americans/Alaskan Natives. These data show that the proportion of Hispanics who presented with distant colorectal cancer increased from 19.8 to 20.3 from 1975 to 1987 to 1988 to 1997; among Native Americans/Alaskan Natives, the increase was more pronounced, from 19.8 to 24.4. More recently published data show similar findings. Using 11 Surveillance, Epidemiology, and End Results cancer registries, Chien et al. (10) showed various racial/ethnic disparities in risk of advanced stage colorectal cancer. One of the strengths of this study is the ability of the investigators to subdivide specific racial/ethnic groups, such as Hispanics, into the various subpopulations. For example, compared to NHWs, men and women of Mexican descent were significantly more likely to present with stage III or IV disease.
Migrant Studies
Wide geographic differences in colorectal cancer rates have been believed to be due in part to environmental factors, namely, dietary intake. Migrant studies have supported these hypotheses, given that individuals moving from countries with low rates to countries with higher rates of colorectal cancer show increased risks similar or close to those of the host country (2,11,12,13,14,15); however, there are some exceptions (16,17). In some instances, the rates of migrants from low-incidence countries exceed those of the host country (11,12,14). For example, colon cancer rates in Japanese living in the United States are currently higher than those of whites. High rates are seen in Caucasian populations of northern European origin, and these high rates continue to be shown with migration. Conversely, where lower rates are seen (i.e., southern Europe, Asia, and Africa), these rates tend to rise with migration to higher rate areas. It has also been shown in recent studies that it is important to take into account length of stay in the host country (13,18). Migrant studies have suggested that colorectal cancer is particularly sensitive to changes in lifestyle/environmental factors. Incidence rates reach those of the host country within one or two generations, sometimes even within the migrating generation, arguing strongly for a nongenetic, environmental etiology.
Colorectal Adenomas
Most colorectal cancers arise from adenomas (19). Because adenomas are usually asymptomatic, they may not be detected until years after onset; thus, the appropriate measure of their frequency is prevalence (e.g., prevalence at the time of endoscopy or autopsy). The prevalence of adenomas increases with age and is greater in men than in women (20). Results of autopsy studies and screening studies of average-risk populations have found that 20% to 60% of individuals have adenomas (20), with the lowest prevalence rates observed in areas of Finland, the Philippines, Mexico, Colombia, Iran, and South Africa (21). Compared to colorectal cancer end points, relatively fewer epidemiologic studies have been conducted using the adenoma as an end point. It is not entirely clear whether lifestyle factors influence adenoma and carcinoma in a differential manner; however, given the complexity of this disease, it is possible that some factors have greater influence in early stages and others in later stages.
Epidemiologic Study Designs
Ecologic or Correlational Studies
The association between colorectal cancer and environmental factors, such as dietary intake, can be assessed using ecologic aggregations by examining correlations between the lifestyle/environmental factor and the corresponding incidence or mortality rates. Such correlational studies, commonly conducted to assess dietary etiology by using per capita consumption data, can be based on comparisons among nations or among administrative units within a single country. It is difficult to draw strong conclusions about cancer etiology based on these studies; nations showing substantial variation in cancer incidence may exhibit, in addition to diverse dietary patterns, differences in nondietary environmental factors. For example, rates of colon cancer are strongly correlated with national per capita disappearance of animal fat and meat, with correlation coefficients ranging between 0.8 and 0.9 (22,23). However, it is difficult to attribute these rates with a high degree of certainty to one or more dietary variables. The primary problem of these correlational studies is that many potential determinants of the cancer of interest, other than the dietary factor under consideration, may vary between areas with high and low incidence rates. Indeed, the number of significant correlates of colon cancer risk may exceed the number of countries under study. These correlates confound one another. Such confounding factors can include genetic predisposition, other dietary factors, and other environmental or lifestyle practices. For this reason, ecologic studies have traditionally been considered the weakest form of epidemiologic evidence.
Analytical Epidemiologic Studies
In case-control studies, information about lifestyle and other factors prior to disease onset is obtained from patients with cancer and compared to those without cancer. Compared to ecologic studies, results from case-control studies may provide stronger evidence given that information on confounding factors can be taken into account. A primary advantage of case-control studies is that these can be conducted over a relatively short period of time, which decreases their cost. An important drawback of case-control studies is the potential for selection or recall bias. Selection bias occurs if an inappropriate control group is selected or if the cases or controls that refuse to participate have characteristics that may bias the results. Recall bias could occur if study participants with a specific cancer remember and report their diet differently from control participants. The possibility of interviewer-induced reporting bias is a consideration, especially if the interviewer is not blinded to the case status of the study participant. Another limitation of such studies is that only dietary factors that are etiologically relevant relatively shortly before the diagnosis of cancer can be practically studied because in the majority of the studies, only diet in the previous year or few years can be assessed. For a disease such as colorectal cancer, in which risk factors from the more distant past appear to be relevant, dietary intake beyond a few years prior to diagnosis may be more relevant; however, difficulty in measuring and validating such dietary intake must be taken into account.
Cohort studies involve identifying a study sample and monitoring the incidence and/or mortality of disease over time as well as exposure to potential risk factors. In these studies, the assessment of exposure factors is obtained before the development of the disease. Thus, the possibility for recall bias that afflicts case-control studies is eliminated. However, a potential drawback related to cohort studies is the loss to follow-up of participants. If disease incidence or specific risk factor exposures are related to a loss to follow-up, then estimates of risk may be biased positively or negatively. Noteworthy associations may not be detected. Cohort studies are generally considered to be expensive; however, inasmuch as several disease end points and intermediate end points can be ascertained in a cohort, these studies can be quite cost effective. When assessing the multicausal nature of various cancers, studies that examine various etiologic factors and outcomes in one data set
are of immense value. For example, studies such as the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) ascertain risk factor data periodically throughout the follow-up period. This allows for the consideration of variation in the risk factor of interest during follow-up. An additional advantage of prospective studies is that biochemical markers can be used, with samples collected prior to the onset of disease. In case-control studies, it is not possible to determine whether the marker reflects true variation in prediagnostic intake or a change related to malignancy.
are of immense value. For example, studies such as the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) ascertain risk factor data periodically throughout the follow-up period. This allows for the consideration of variation in the risk factor of interest during follow-up. An additional advantage of prospective studies is that biochemical markers can be used, with samples collected prior to the onset of disease. In case-control studies, it is not possible to determine whether the marker reflects true variation in prediagnostic intake or a change related to malignancy.
Lifestyle and Environmental Factors
Notwithstanding the importance of genetic influences, several lines of evidence support the substantial role of lifestyle/environmental factors in the etiology of colorectal cancer. Unlike other malignancies, risk of colorectal cancer appears to be influenced by a variety of modifiable factors, ranging from those that are relatively easy to quantify (i.e., tobacco exposure) to those whose assessment is much more challenging and complex (i.e., dietary intake, physical activity, and environmental/occupational exposures). This section presents lifestyle and environmental risk factors that are currently believed to have an important role in the development of colorectal neoplasia.
Obesity
The majority of epidemiologic studies supports a role for obesity as a risk factor for colorectal adenoma (24,25,26,27), cancers (25,28,29,30,31,32,33), and colon cancer mortality (29,34). These observations suggest a continuous action of the adverse effects of obesity along the adenoma to carcinoma continuum. In general, the effect of obesity on colorectal cancer risk has been stronger for cancers arising in the colon, for those occurring in men (30,35,36,37,38,39,40), and for cancers in the proximal colon (30,33). In the Physician’s Health Study (PHS), a large cohort of male health professionals, the relative risk (RR) for colon cancer was 1.48 among men in the upper quintile of body mass index (BMI) compared to those in the lowest quintile (p = 0.02) (25). In NHS, a large cohort study of women, a BMI >29 kg/m2 was associated with a RR of 1.45 (95% CI, 1.02–2.07) when compared to a BMI <21 kg/m2 (41). Further recent findings from the prospective Framingham Cohort study, where waist circumference and waist-to-hip ratio (WHR) measures were available, suggest that measures of central adiposity may be more informative for risk of colon cancer than measures of BMI (30). In the Framingham study, a large waist circumference was associated with a RR for colon cancer of 4.4 for middle-age adults and 3.0 for older sedentary adults (30). Earlier data from the HPFS reported similar findings where stronger associations were observed with measures of waist circumference (RR, 2.6) and WHR (RR, 3.5) than for BMI (25).
Studies among women have been less consistent, with the majority of studies reporting weak or null associations between measures of BMI and colon cancer risk and strong effect modification by menopausal status (39). The use of waist circumference as a measure of central obesity may improve the precision of point estimates for colon cancer risk among women (30,41,42,43). Stronger effects of high BMI on colorectal cancer among premenopausal versus postmenopausal women (39,44) has led to the suggestion of effect modification by menopausal status. The consistent reporting of protective effects of hormone replacement therapy for colorectal cancers in postmenopausal women (see “Hormone Replacement Therapy” section), and attenuation of colorectal cancer risk among heavier older women may indicate a physiological shift from obesity as a risk-enhancing to a risk-reducing factor as women age. Although exposure to higher levels of growth-promoting effects of insulinlike growth factor 1 are proposed as an explanation of the menopause-specific effects of obesity on colorectal cancer risk (39), further study is needed to delineate the mechanism for the observed attenuation of risk for colorectal cancers among heavier, older women.
Physical Activity
Results of prospective (41,45,46,47,48,49,50,51,52,53,54,55) and retrospective (56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77) studies support an inverse association between physical activity and risk of colon but not rectal cancer (46,50,51,53,56,69,78,79). The results are consistent, whether assessing active versus nonactive or sedentary versus active individuals. In a prospective study of female nurses (41), leisure-time physical activity and body size were assessed in relation to the subsequent development of colon cancer. Women in the upper quintile of physical activity exhibited approximately half the colon cancer risk of inactive women (RR, 0.54; 95% CI, 0.33–0.90). These findings are supported by results of other published studies, including those of the HPFS (25). When physical activity and BMI are assessed jointly, the highest risk of colon cancer occurs among those who are both physically inactive and have high BMI levels (25,30,80). The joint adverse effect of obesity and sedentary behavior is demonstrated for both men and women (30).
Despite the wide variation in physical assessment methodology among studies, including type of activity (leisure-time or occupational) and method of assessment, considerable consistency between studies is found. Based on a comprehensive review of the literature, Colditz et al. (81) reported a dose–response protective effect of physical activity on colon cancer with approximately a 50% reduction in incidence of colon cancer among individuals with the highest level of physical activity. In addition, a recent meta-analysis of 19 cohort studies (82) supports an approximate 30% significant reduction in the risk of colon cancer in physically active males and females with no protective effect of physical activity on rectal cancers.
The proposed biological mechanisms for the effect of physical activity on colon carcinogenesis are numerous and not necessarily mutually exclusive. The protective action of aspirin and other NSAIDs in the colon suggest that proinflammatory prostaglandins play an important role in the development of colon cancer (83,84,85,86). Martínez et al. (87) found a strong inverse association between physical activity and rectal mucosal PGE2 concentrations. These results suggest that one potential biological mechanism by which physical activity alters the risk of colon cancer is through direct effects on local PGE2 and other prostanoid synthesis.
Other mechanisms proposed to explain the protective effect of physical activity in the colon include decreased bowel transit time with concomitant reduction in exposure to dietary carcinogens, lower bile acid secretion, and maintenance of insulin sensitivity and glucoregulatory function (88). Chronic insulin exposure or hyperinsulinemia coupled with impaired insulin sensitivity is perhaps the best supported of the mechanisms and provides a strong link between the main colon cancer risk factors of age, inflammation, physical inactivity, and central adiposity (89).
Hormone Replacement Therapy
Although studies on reproductive factors and colorectal cancer risk have provided conflicting results, HRT use has been consistently associated with a decreased risk of colon cancer with weaker or null effects in the rectum in the majority
of case-control and cohort studies (45,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104). In the NHS (105), current postmenopausal HRT use was associated with a decreased risk of colorectal cancer; the relationship with any past use was weaker and absent 5 years after hormone use was stopped. These findings, along with two additional large prospective studies (106,107), support an inverse association between colon cancer and estrogen use in postmenopausal women. A large multicenter case-control study (108) also indicated that women who had ever used HRT had a lower colon cancer risk (OR, 0.82; 95% CI, 0.67–0.99), with recent use associated with approximately 30% reduction in risk (OR, 0.71; 95% CI, 0.56–0.89). Results of another large case-control study (109) also found an inverse association between HRT and colorectal cancer, particularly among recent users (RR, 0.54; 95% CI, 0.36–0.81). Overall, the results of recent studies show inverse associations ranging from 0.5 to 0.8 for HRT use. A meta-analysis of 18 studies conducted to assess the role of HRT and colorectal cancer (91) found an overall 20% reduction in risk for colon cancer associated with every use of HRT (RR = 0.80; 95% CI = 0.74–0.86). These results were not appreciably different for rectal cancers (RR, 0.81; 95% CI, 0.72–0.92). Furthermore, the association with colorectal cancer was strongest among current HRT users (RR, 0.66; 95% CI, 0.59–0.74).
of case-control and cohort studies (45,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104). In the NHS (105), current postmenopausal HRT use was associated with a decreased risk of colorectal cancer; the relationship with any past use was weaker and absent 5 years after hormone use was stopped. These findings, along with two additional large prospective studies (106,107), support an inverse association between colon cancer and estrogen use in postmenopausal women. A large multicenter case-control study (108) also indicated that women who had ever used HRT had a lower colon cancer risk (OR, 0.82; 95% CI, 0.67–0.99), with recent use associated with approximately 30% reduction in risk (OR, 0.71; 95% CI, 0.56–0.89). Results of another large case-control study (109) also found an inverse association between HRT and colorectal cancer, particularly among recent users (RR, 0.54; 95% CI, 0.36–0.81). Overall, the results of recent studies show inverse associations ranging from 0.5 to 0.8 for HRT use. A meta-analysis of 18 studies conducted to assess the role of HRT and colorectal cancer (91) found an overall 20% reduction in risk for colon cancer associated with every use of HRT (RR = 0.80; 95% CI = 0.74–0.86). These results were not appreciably different for rectal cancers (RR, 0.81; 95% CI, 0.72–0.92). Furthermore, the association with colorectal cancer was strongest among current HRT users (RR, 0.66; 95% CI, 0.59–0.74).
More recently, evidence from the published findings of the large randomized clinical trial of HRT conducted in older postmenopausal women by the Women’s Health Initiative (WHI) supports previous findings from case-control and cohort studies for a protective effect of HRT in the colon. Among women taking combination HRT (estrogen combined with progestin), the WHI reported 43 cases of invasive colorectal cancers in the treatment group and 72 in the placebo group (HR, 0.56; 95% CI, 0.38–0.81) (110). In contrast, among all users of estrogen only, there were no differences in incidence of colorectal cancers when compared to placebo in unpublished early reports (111). Notable for women taking combination HRT therapy, among those developing colorectal cancers, the disease was significantly more likely to present with lymph node or regional/distant metastasis compared to placebo; features associated with poor outcomes (110). The significant association of combination HRT with advanced disease at diagnosis was unexplained, but its observation early in the trial suggest either a promoting effect of the hormonal exposure on the growth of existing malignancies or perhaps a masking effect of early symptoms of disease and delayed diagnosis. Taken together, the evidence is highly compelling for HRT use as beneficial for the prevention of colon cancers, with some caution warranted for active surveillance among users (110,112). Given potential limitations of the WHI randomized trial study population, most notably the older age of subjects at initiation of HRT use, additional studies to estimate the overall benefit of HRT in younger postmenopausal women is warranted.
Tobacco
Although tobacco has not been clearly implicated as a cause of colorectal malignancies, a higher risk of adenomatous polyps has been consistently observed among smokers in numerous studies (113). A long induction period between smoking and risk of colorectal cancer was hypothesized based on results from two large cohort studies (114,115). Subsequently, the vast majority of published studies have reported positive associations between cigarette smoking and colorectal cancer (45,116,117,118,119,120,121,122,123,124,125), although several studies did not support an association (126,127,128,129). Of note, some of the nonsupportive studies were conducted in Sweden (127,128), suggesting that some factor, possibly genetic, in Swedes may counter the impact of smoking. In an earlier review of the published data, Giovannucci and Martínez (113) suggested that the evidence earlier in the decade tended not to support the hypothesis that smoking influenced colorectal carcinogenesis because a sufficient lag period had not elapsed between smoking and colorectal cancer risk. With the assumption that an increased risk emerges only about four decades after smoking initiation, a pattern consistent with a positive association emerges. The consistent finding of a positive association between smoking and the risk of adenomas probably results from a presumably much shorter induction period for these lesions. Based on this earlier review of the literature, Giovannucci and Martínez (113) concluded that overall evidence supports the hypothesis that tobacco smoke is an initiator of colorectal carcinogenesis and that the induction period is very long, possibly up to four decades.
A more recent review of the literature conducted by Giovannucci (130) indicates that data published since the 1990s strongly supports the association between tobacco and colorectal neoplasia. For colorectal adenoma, there is a high degree of consistency in the findings across studies reporting an association with tobacco smoke, with risk estimates ranging between 2 and 5. In a review of colorectal cancer studies conducted after 1970, which would allow for long-term exposure, all 10 studies conducted in the United States support an association with tobacco smoke. Given that women in the United States took up smoking later than men in the United States, a sufficient induction period would only be observed after 1990. As a result, a review of studies published after 1990 also shows that all five of these support this association; data from recent studies outside the United States also show positive associations. In his review, Giovannucci advocates for the inclusion of colorectal cancer in the list of tobacco-associated malignancies. Due to the hypothesized potential causal nature of this association, population-attributable risk estimates have been provided in the literature, which range from 11% for colon cancer in women (118) to 22% of rectal cancer in men (131). This implies that if the association between tobacco and colorectal cancer is causal, 11% to 22% for these malignancies are attributable to this particular exposure.
Nonsteroidal Anti-inflammatory Drugs
As reviewed by Thun et al. (132), a lot of support exists for a protective effect of NSAID use and COX-2 inhibitors on colorectal neoplasia. Evidence in favor of an inverse association between NSAID use (e. g., aspirin, indomethacin, ibuprofen, piroxicam) and colorectal cancer stems from epidemiologic (133,134,135,136,137), animal (138,139,140,141), and intervention studies among individuals with familial adenomatous polyposis (142,143,144). In addition, patients with rheumatoid arthritis, who generally have higher use of NSAIDs, have lower incidence and mortality rates of gastrointestinal malignancies (145,146). Supporting evidence is also derived from observational studies of NSAIDs and colorectal adenomas (127,137,138,139).
Results of epidemiologic studies are consistent with an approximately 50% reduction in colorectal cancer risk associated with use of aspirin or other NSAIDs, although in one study a positive association was observed (147). In the NHS, a statistically significant reduction of colorectal cancer in women after 20 years of consistent (two or more tablets per week) aspirin use was observed (RR, 0.56; 95% CI, 0.36–0.90; p = 0.008) (137). Similarly, a prospective study in male health professionals reported relative risks of comparable magnitude (136). Perhaps the strongest evidence of a protective effect of NSAIDs for sporadic colorectal neoplasia was provided by a randomized, double-blind clinical trial conducted by Baron et al. (148). Results from this study revealed a significantly reduced risk for colorectal adenoma in those randomized to receive 81 mg/day
of aspirin compared to the placebo group, although no effect was found for an aspirin dose of 325 mg/day (148).
of aspirin compared to the placebo group, although no effect was found for an aspirin dose of 325 mg/day (148).
Despite these encouraging results, the use of COX-2 inhibitors for chemoprevention of colorectal neoplasia in the general public is unlikely in the near future. The Vioxx Gastrointestinal Outcomes Research trial, designed to test the effects of rofecoxib on gastrointestinal outcomes, reported significant increases in adverse cardiovascular events related to the use of rofecoxib (149). This led to the termination of several other ongoing trials of COX-2 inhibitors and colorectal neoplasia. Therefore, despite a wealth of information indicating protection from colorectal adenoma and cancer by COX-2 inhibitors, the future use of these compounds as preventive agents for sporadic colorectal adenoma and cancer is uncertain.
Diet
It has been frequently cited that up to 90% of cancer is environmentally related (150,151). Diet constitutes one of the most obviously differential population parameters in that it involves a daily exposure that is highly adaptable to a new environment. The epidemiologic and experimental evidence that dietary pattern is an important causal determinant of colorectal tumors is compelling. However, controversy exists regarding the specific nutrients, foods, or combinations of these that are causally related to the development of colorectal cancer. Specifically, hypotheses that were prevalent a decade or two ago remain controversial despite intensive investigation.
Long-term diet is usually the exposure of interest for epidemiologic studies of cancer. An assessment that takes into account day-to-day variation in dietary intake is essential when choosing an appropriate assessment method. Therefore, dietary instruments that measure only 1 or a few days can result in substantial misclassification of an individual’s true long-term intake. The inability to reduce this error can result in an attenuation of relative risks, that is, to lower the strength of the associations (152,153). High intraindividual variation of micronutrient intake is possible given wide differences of individual nutrient concentration in certain foods (154). In the same context, fruit and vegetables may only be abundant during a certain part of the year, resulting in additional variation. Cultural and socioeconomic influences may also play a role in the variation of dietary intake. Given these complexities, it is important to note that all dietary assessment methods are prone to measurement error that may arise from various sources. Consequently, individuals may be classified in the wrong intake categories. This misclassification in turn weakens observed associations.
As noted previously, long-term dietary intake is of relevance in epidemiologic studies of cancer. As a result, the food frequency questionnaire (FFQ) approach has been the preferred instrument of assessment. Rather than obtaining a more precise but likely unrepresentative estimate of short-term dietary intake, the FFQ seeks to measure average, long-term intake. Most FFQs focus on the preceding year (or the year prior to diagnosis of cancer for case-control studies) as the period of exposure. The basic components of the FFQ include a food list and a frequency response section. Other questionnaires also incorporate a section related to usual portion size. Because FFQs are relatively easy to administer, they are considered to be extremely practical in epidemiologic settings. Unlike other dietary instruments such as diet records, processing of large numbers of FFQs is feasible and relatively inexpensive.
However, FFQs are not without weaknesses for studies of diet and cancer. Major criticisms of this method include the inability of study participants to recall long-term food intake patterns, poor validity of instruments, and inability to detect diet–cancer relationships as compared to dietary biomarkers or food records (155). Suggestions for improvements to FFQs include measurement of dietary behavior as well as food intake, and collection of real-time information via computers (155).
Energy Intake
The assessment of the relationship between energy intake and colon cancer presents a challenge because total energy is correlated with both “good” and “bad” nutrients (e. g., folate, calcium, red meat) and nonnutrient factors (e. g., physical activity, obesity), which themselves have been implicated in colon cancer risk. Variation in energy intake among individuals within a population is influenced largely by level of physical activity, metabolic efficiency, and body size (156). Therefore, the confounding effects of these factors on total energy intake should be taken into account when examining the role of dietary factors on the risk of developing colorectal cancer.
Results of most published case-control studies have shown a positive association between total energy intake and risk of colon cancer (56,59,65,157,158,159,160,161,162,163,164,165,166). Howe et al. (167) conducted a pooled analysis of 13 case-control studies and found that total energy intake was associated with a higher risk of colon cancer, regardless of whether the energy source was fat, protein, or carbohydrate. Slattery et al. (80) reported similar findings based on three case-control studies and suggested that total energy intake is more important than the specific energy sources. In contrast to the findings of case-control studies, cohort studies have shown no relationship or even a slight inverse association between total energy intake and risk of colon cancer (168,169,170,171,172,173). In one of these studies (173), a statistically significant RR of 0.62 was reported between high energy intake and colorectal cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree