Fig. 7.1
Laparoscopic sleeve gastrectomy. Reprinted with permission from Parikh M, Gagner M, Pomp A. Laparoscopic Duodenal Switch. In: Nguyen NT, De Maria EJ, Ikramuddin S, Hutter MM. eds. The SAGES Manual: a Practical Guide to Bariatric Surgery. Springer, New York, 2008;109–129 [38] © Springer
Short-term weight loss comparable to that of the gastric bypass (60–70 % excess weight loss by 3 years)
Improvement in insurance coverage for the LSG
Favorable complication profile compared to the gastsric bypass
Less required postoperative follow-up compared to gastric banding
Surgeons experienced with LSG report that the most common complications include leak, hemorrhage, stenosis, spleen/liver injury, portal vein thrombosis, and reflux [2]. This chapter focuses on leak after LSG, with a particular focus on prevention and management.
7.2 Presentation and Diagnosis
7.2.1 Incidence and Presentation
The rate of staple-line leaks after LSG varies in the literature, but is generally between 1.1 and 5.3 % of cases [3]. A systematic review of 9991 LSG reported a leak rate of 2.2 % [4]. The mortality rate from leaks after LSG is 0.11 % [4]. The vast majority of leaks (75–89 %) occur proximally, near the gastroesophageal junction [5].
Leaks present at a mean of 7 days postoperatively, but can present as late as 120 days postoperatively [3]. The majority of leaks present after patients are discharged home from the hospital; therefore close follow-up in the immediate postoperative period is critical after LSG. Rosenthal et al. proposed a classification system for leak after LSG based on timing: acute leak (within 7 days postoperatively), early leak (within 1–5 weeks postoperatively), late leak (greater than 6 weeks postoperatively), and chronic leak (after 12 weeks) [6].
Staple-line leak after LSG can present with many clinical scenarios, ranging from a stable patient with mild abdominal pain to a patient with manifestations of systemic inflammatory response syndrome (SIRS—see Table 7.1) to a patient with sepsis and multiorgan failure. A high index of suspicion is important, as early intervention is the key to successful management of these patients [7].
Table 7.1
SIRS criteria based on Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP)
SIRS criteria | |
|---|---|
Presence of two of the following: | |
• Temperature >100.4 ° F or < 96.9 ° F • WBC > 12,000 or <4000 or >10 % bands • HR >90 bpm • RR >20, PaCO2 < 32 • Gap acidosis |
7.2.2 Diagnostic Study
Abdominal computed tomography (CT) scan with oral and intravenous contrast is the diagnostic study of choice for most patients suspected of having leak. CT findings may range from blips of extraluminal air to frank contrast extravasation (Figs. 7.2 and 7.3). Esophagrams may also be used to diagnose leak; however it may be normal despite the presence of leak.
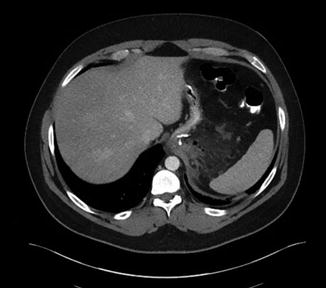
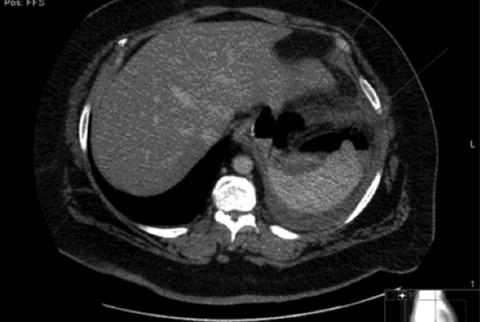

Fig. 7.2
Blips of air around staple line in patient POD#9 after LSG. This resolved with intravenous antibiotics

Fig. 7.3
CT scan POD#8 showing extraluminal fluid collection consistent with leak
Since leaks often present after patient discharge from the hospital, the value of immediate postoperative upper GI studies has been debated. Studies have demonstrated the lack of association between routine postoperative swallow study and leak [8]. Similarly, intraoperative leak tests fail to detect leak, unless due to a stapler misfire or other technical error. A normal intraoperative leak test and a normal postoperative swallow study do not preclude the development of staple-line leak after LSG. Despite this, many surgeons still favor these tests and perform them routinely.
Some surgeons also advocate for routine drain placement after LSG. However, this has fallen out of favor as leaks present nearly a week after LSG, and leaving a drain in for this duration is unnecessary in a vast majority of LSG cases. In 2013, 39 % of surgeons left a drain in the abdominal cavity after LSG, and this number continues to decline [2]. If a drain is left in place, however, postoperative leak test with methylene blue may be effective in diagnosing leak. Some surgeons have also used this method during follow-up to monitor the progress of the fistula [9, 10].
7.3 Prevention of Leak After LSG
Leak after LSG can occur for a variety of reasons. Possible factors include patient-level factors that predispose to leak. Other factors may be related to the technical aspects of LSG construction, inadequate oxygenation with subsequent ischemia, or thermal injury [11].
7.3.1 Patient Characteristics
Certain patient factors may be associated with increased leak rate. Benedix et al. retrospectively reviewed 103 leaks in 5400 LSG cases (1.9 %) performed over a 6-year period in order to identify factors that increase the risk of leak [12]. They found that higher body mass index (BMI), male gender, presence of sleep apnea, conversion to laparotomy, longer operative time, year of procedure, and intraoperative complications significantly increased leak rate. On multivariate analysis, however, only operative time and year of procedure maintained a significant association with leak.
Superobese patients (BMI >50 kg/m2) may have a higher incidence of leak, as is the case in gastric bypass. A systematic review of 4888 LSG found the leak rate to be 2.9 % among the superobese versus 2.2 % in those with a preoperative BMI <50 kg/m2, but this was not statistically significant [5]. Another study found type 2 diabetes to be an independent risk factor for the development of leak (p < 0.01) [13].
Sakran et al. found an association between previous bariatric surgery and increased likelihood of leak (p < 0.005). Leaks developed in 44 out of 2834 LSG (1.5 %). Eleven patients (25 %) had a prior silastic ring vertical gastroplasty or LAGB, versus 10 % of non-leaks, implying a threefold increased risk of leak in patients with previous bariatric surgery [3].
7.3.2 Technical Factors
In a retrospective review of 529 cases with 0 % leak rate, Bellanger et al. discussed the technical principles for decreasing enteric leakage after LSG [14]. A key point mentioned is to position the tip of the stapler to give a distance of one and a half times the width of the bougie at the area of the incisura angularis (Fig. 7.4). Other technical principles included positioning the stapler to leave 1 cm of gastric tissue lateral to the angle of HIS to avoid stapling too close to the esophagus in the area of the cardia (Fig. 7.5), allowing adequate compression of the gastric tissue with the stapling device, and thorough visual inspection of the staple line after procedure completion [14].
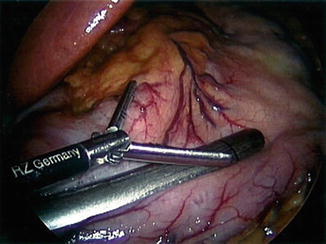
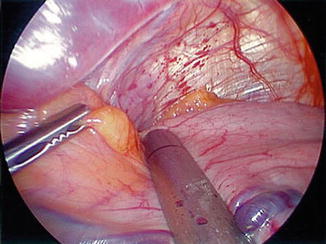

Fig. 7.4
First application of stapler one and a half times the distance from the bougie. Reprinted with permission from Bellanger et al. Laparoscopic sleeve gastrectomy, 529 cases without a leak: short-term results and technical considerations. Obesity Surgery 2011;21:146–50 [14] © Springer

Fig. 7.5
Application of stapler lateral to periesophageal fat pad. Reprinted with permission from Bellanger et al. Laparoscopic sleeve gastrectomy, 529 cases without a leak: short-term results and technical considerations. Obesity Surgery 2011;21:146–50 [14] © Springer
Sakran et al. proposed that heat-producing instruments may cause thermal injury to the sleeve, leading to leak. Additionally, aggressive dissection near the posterior aspect of the upper sleeve may cause devascularization, increasing susceptibility to leak. They propose that dissection in this area should be kept to a minimum and the final staple fire should be directed away from the esophagus and to the left of the gastroesophageal junction [3].
7.3.3 Systematic Review and Meta-Analysis of Factors That Contribute to Leak (Table 7.2)
Table 7.2
GEE (general estimating equation) model adjusting for the effect of bougie size, distance from pylorus, and the use of buttressing or sutures on leak rate while controlling for age and BMI
Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
OR | 95 % CI | p-Value | OR | 95 % CI | p-Value | |
Bougie size | ||||||
<40 Fr (reference) | – | – | ||||
40–49 Fr | 0.69 | [0.41, 1.16] | 0.161 | 0.53 | [0.37, 0.77] | 0.0009 |
≥50 Fr | 0.37 | [0.18, 0.73] | 0.0041 | 0.40 | [0.15, 1.07] | 0.068 |
Distance to pylorus | ||||||
<5 cm (reference) | – | – | ||||
≥5 cm | 1.16 | [0.60, 2.25] | 0.659 | 1.30 | [0.81, 2.09] | 0.279 |
Use of buttressing/sutures | ||||||
Bioabsorbable (reference) | – | – | ||||
No buttressing, no sutures | 1.00 | [0.37, 2.69] | 0.997 | 1.06 | [0.49, 2.30] | 0.873 |
Non-absorbable buttressing | 1.78 | [1.17, 2.72] | 0.0075 | 2.01 | [0.87, 4.68] | 0.104 |
No buttressing, sutures only | 1.95 | [1.25, 3.02] | 0.0031 | 2.87 | [1.21, 6.84] | 0.017 |
Age | ||||||
Mean age < 40 | – | |||||
Mean age 40–44 | 0.78 | [0.51, 1.19] | 0.250 | 0.83 | [0.54, 1.27] | 0.392 |
Mean age 45+ | 0.51 | [0.27, 0.98] | 0.044 | 0.57 | [0.31, 1.03] | 0.061 |
BMI | ||||||
Mean BMI < 45 | – | |||||
Mean BMI 45–49 | 1.82 | [0.99, 3.32] | 0.052 | 1.81 | [1.21, 2.71] | 0.0041 |
Mean BMI 50+ | 1.44 | [0.73, 2.84] | 0.296 | 1.96 | [1.16, 3.34] | 0.012 |
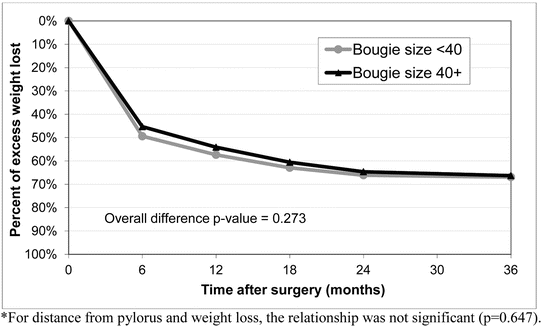
Technical aspects of LSG, including bougie size used to calibrate the sleeve, distance from the pylorus where the stapling begins, height of stapler used to transect the stomach, and the role of buttressing material on the staple line, may affect leak rate. Debate exists whether the creation of tighter (i.e., smaller) sleeves results in higher leak rate (Fig. 7.6) [15].
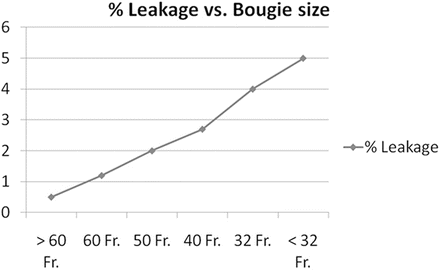

Fig. 7.6
Percentage of leakage versus bougie size. On the x-axis, a bougie size in French and on the y-axis leakage rate in percentage. Reprinted with permission, Gagner M. Leaks after sleeve gastrectomy are associated with smaller bougies. Prevention and treatment strategies. Surg Laparoscopic Endosc Percutan Tech 2010;20:166–169 [15] © Wolters Kluwer Health
In a meta-analysis of 9991 LSG, various technical aspects of performing LSG were analyzed [4]. Bougie size was <40 Fr in the majority (69 %) of patients, LSG transection began ≥5 cm from the pylorus in 68 % of patients, and some form of buttressing was used in 82 % (Fig. 7.7). All leaks were analyzed based on bougie size, the distance from the pylorus, the use of buttressing, and the type of buttressing (Fig. 7.8).
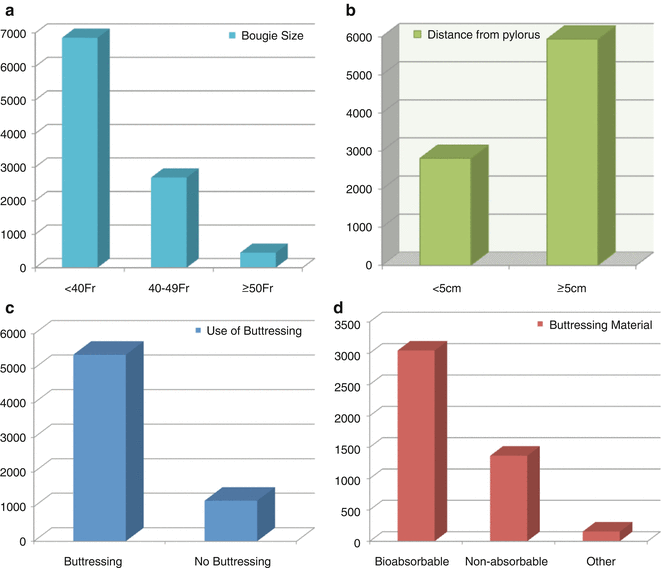
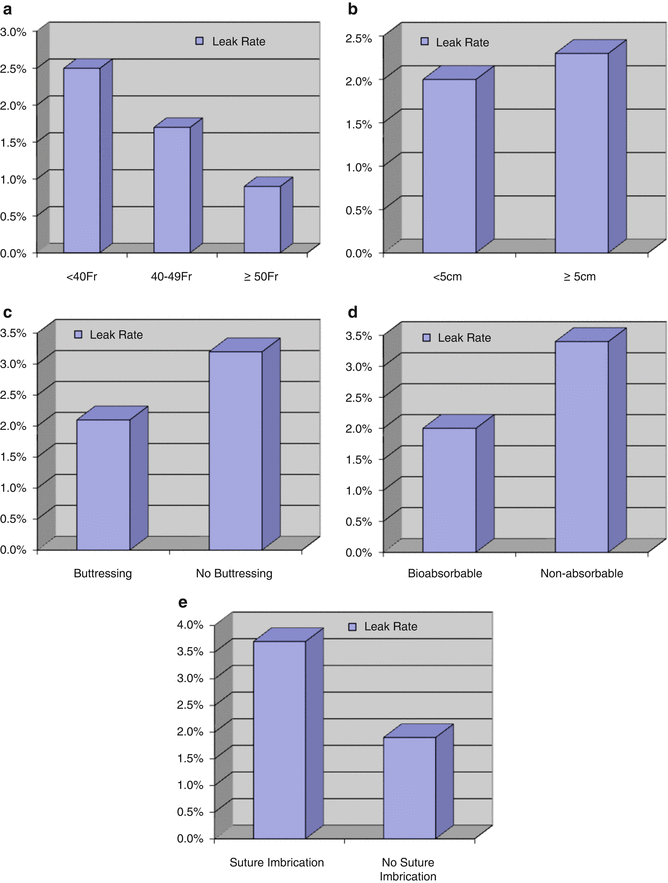

Fig. 7.7
Most common techniques used for LSG. Reprinted with permission from Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy. Ann Surg 2013;257:231–237 [4] © Wolters Kluwer Health

Fig. 7.8
Effect of technique on leak rate. Reprinted with permission from Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy. Ann Surg 2013;257:231–237 [4] © Wolters Kluwer Health
Due to the fact that there are multiple factors that may contribute to leak, a general estimating equation (GEE) model was then created utilizing the variables of bougie size (<40 Fr, 40–49 Fr, ≥50 Fr), distance from the pylorus (<5 cm, ≥5 cm), and the use of buttressing (bioabsorbable, non-absorbable, other, none) while controlling for age and BMI. The GEE model revealed that the risk of leak after LSG decreased by using a bougie ≥40 Fr (OR 0.53 [0.37–0.77], p = 0.0009; see Table 7.2). Distance from pylorus did not impact leak rate (p = 0.279). The use of bioabsorbable buttressing did not impact leak rate (p = 0.104). However suturing alone (without buttressing) increased leak (OR 2.87 [1.21–6.84], p = 0.017). BMI > 50 also increased leak rate (OR 1.96 [1.16–3.34], p = 0.012). A linear repeated measures regression model was used to compare weight loss between bougie size <40 Fr and bougie size ≥40 Fr and found no difference in weight loss up to 3 years (70.1 % mean EWL; p = 0.273) (Fig. 7.9). Based on this study, one of the most important technical factors that may decrease leak is utilizing bougie ≥40 Fr.