Chapter 27 Endoscopic Treatment of Superficial Esophageal Cancers
Biology of Early Esophageal Cancer
In contrast to Asia, where the high incidence of esophageal cancer has led to screening programs, esophageal cancer in the West has primarily been found when patients become symptomatic. Although it has been established that older white men with reflux symptoms are at greatest risk of esophageal adenocarcinoma and Barrett’s esophagus, the high prevalence of Barrett’s esophagus in patients without symptoms makes screening for this condition in only symptomatic patients problematic.1,2 Surveillance endoscopy finds patients with earlier staged malignancies that are suitable for endoscopic therapies.3
Cancers related to Barrett’s esophagus apparently arise from chronically inflamed areas of the mucosa. The mechanism of carcinogenesis seems to be related to inflammation producing increased levels of prostaglandin E2, which is an inflammatory mediator that can cause increased cell proliferation.4 Cell proliferation has been used as a marker of neoplasia and is an early event in cancer development. Increased cell proliferation drives the cell cycle, and progression to cancer in Barrett’s esophagus usually involves the loss of cell cycle checkpoint gene products such as p16. The loss of p16 function through either promoter inactivation via hypermethylation or loss of heterozygosity occurs early in carcinogenesis and is found almost universally in dysplastic Barrett’s mucosa.5 This loss of cell cycle control leads to further acceleration of the cell cycle, which allows further genetic events to occur including loss of p53, which is well recognized to be an important tumor suppressor gene that is also involved in promoting apoptosis of cells that have accumulated genetic defects.6 The loss of p53, which is important for elimination of cells with chromosomal abnormalities, and the increase in proliferation of cells caused by the loss of p16 lead to further chromosomal instability, which is manifested by the appearance of aneuploidy, or abnormal amounts of DNA.7 These large-scale changes of chromosomal loss are related to the degree of histologic changes of dysplasia in the tissue with minimal DNA loss in nondysplastic tissue, moderate loss in low-grade dysplasia, and large degrees of loss in patients with high-grade dysplasia or cancer.
The important aspect of the biology of Barrett’s esophagus to the endoscopist is that these genetic changes do not always correlate with histologic changes, especially when ablative therapies have been applied. Ablative therapy has the ability to decrease histologic changes of dysplasia, while allowing genetic abnormalities to persist.8 These persistent genetic abnormalities over time lead to recurrence of dysplasia and cancer. These findings suggest that histologically benign Barrett’s esophagus after ablative therapy may be precancerous, and long-term control of the neoplastic risk in Barrett’s esophagus may involve elimination of any of the tissue with genetic abnormalities.
These findings were verified by data from a randomized prospective study of photodynamic therapy for Barrett’s esophagus with high-grade dysplasia. In this study of 208 patients randomly assigned to either photodynamic therapy in combination with omeprazole or omeprazole alone, patients in whom complete elimination of Barrett’s mucosa was achieved did not progress to cancer, whereas patients with any residual Barrett’s mucosa did have a significant chance of developing cancer.9 The complete elimination of the intestinal metaplasia may be difficult even with newer therapies such as radiofrequency ablation; in a randomized trial of patients with low-grade and high-grade dysplasia, the dysplasia could be eliminated in greater than 90% of patients, but the intestinal metaplasia remained in 77%.10
Staging of Early Esophageal Cancer
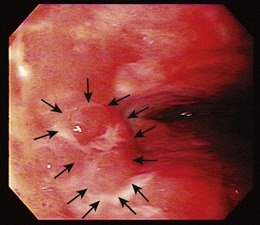
Endoscopic treatment of early esophageal cancer must involve careful and accurate staging of the malignancy. Previously, early esophageal cancer was primarily defined endoscopically on the basis of size. An example of an early cancer in Barrett’s esophagus is shown in Fig. 27.1. Cancers that were 2 cm or less in diameter were generally thought to be early cancers.11 This definition was inaccurate because visualization of local lymph nodes and assessment of the depth of tumor invasion were impossible without surgical resection during this time period. However, surgical resections have shown physicians that early mucosally based cancers above the muscularis mucosae are rarely associated with metastatic disease. In a survey of European centers that performed 253 esophagectomies for early squamous cell carcinomas, it was found that patients with disease confined to the epithelium had a survival rate of 92.8%.12 With penetration into the submucosa, the 5-year survival rate decreased to 72.8%. The overall mortality rate for esophagectomy for early cancers in this series was 9.1%. Similar results for squamous cell carcinomas were reported from Japan, where there was increased chance of metastasis if squamous cell carcinoma was found to penetrate beyond the muscularis mucosae.13
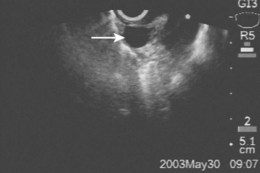
The advent of endoscopic ultrasound (EUS) has affected the need for surgical resection to define the depth of cancer invasion accurately. EUS can be performed with either dedicated echoendoscopes or high-frequency ultrasound probes. The echoendoscopes allow the endoscopist to visualize at 7.5- to 12-MHz frequencies, which permits examination of the periesophageal lymph nodes (N1 stage), celiac lymph nodes (M1a stage), nodes of the lesser curve of the stomach (N1 stage), most of the liver (M1b stage), and pleural or vascular involvement (T4 stage) by the tumor. An example of a periesophageal lymph node on EUS is shown in Fig. 27.2. A lymph node is suspicious on EUS if it is hypoechoic, rounded, in proximity to the tumor, and more than 1 cm in diameter. If suspicious lymph nodes are found, they should be sampled by fine needle aspiration performed using a linear array instrument.
For mucosally based lesions, ultrasound probes are more desirable because they can image at 20 to 30 MHz allowing the endoscopist to image at the resolution needed to resolve the muscularis mucosae to a greater extent. In addition, the use of probes allows visualization using water filling the esophagus, which is necessary to view small mucosal cancers without the artifact caused by the compression by a balloon that is traditionally used with echoendoscopes. Ultrasound probes cannot image deep into the tissue to visualize lymph nodes, however, so to stage an early cancer accurately, both probes and echoendoscopes are needed.14 Despite the use of careful ultrasound examination, lymph nodes can be missed or inaccessible because of Barrett’s mucosa that is in the field of view. It is believed that false-positive findings can be caused by contamination with epithelial cells.15
Small case series have been performed of patients with Barrett’s esophagus and early cancers staged by EUS before esophagectomy. At one center, the technique was 100% sensitive for detection of submucosal invasion but was only 90% specific for invasion in a group of 22 patients.16 Inflammatory changes are virtually impossible to differentiate from early cancer invasion by EUS techniques. Overall, EUS seems to have a tendency to overstage the depth of tumor invasion. A retrospective review of the experience of a single center with EUS staging of esophageal cancer from 1991–2001 in 222 patients found that the accuracy of EUS in tumor staging was only 54%, and the accuracy of EUS in lymph node staging was 65%.17 These results did not seem to be related to a “learning curve” in EUS interpretation because the accuracy in the first half of the time period studied was similar to the accuracy in the second half of the study period. More recent publications emphasize that although EUS may not be as accurate as previously hoped in staging, the information provided from having a negative evaluation for lymph nodes or for resectability was nonetheless valuable.14
Endoscopic mucosal resection (EMR) has been used to help define the depth of cancer penetration and as a primary treatment for early cancer in Barrett’s esophagus. EMR involves the use of a friction fitted cap that attaches to the tip of a standard endoscope. This technique was pioneered in Japan, where EMR has become the standard of care for early mucosal esophageal cancers. A survey of more than 145 Japanese hospitals found that 76% used EMR for the treatment of early esophageal cancers.18
The cap technique is the predominant technique used in the United States because of its commercial availability. The cap is available in the two styles shown in Fig. 27.3; one is completely level, whereas the other has the lip of the cap at an oblique angle. The cap also varies in terms of the consistency of the material from which it is made. Generally, hard plastic caps are favored when there is a need to try to suction more scarred tissue, whereas soft caps are more useful when passing the caps through the upper pharynx. The oblique cap is favored in resection of larger pieces of tissue, whereas the straight cap seems to be more favored when precision is required in the amount of tissue necessary for resection.
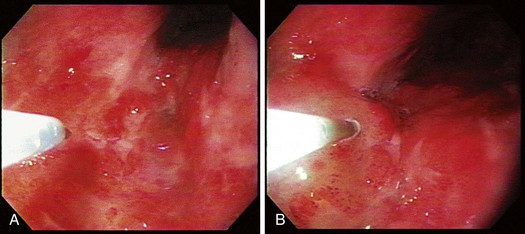
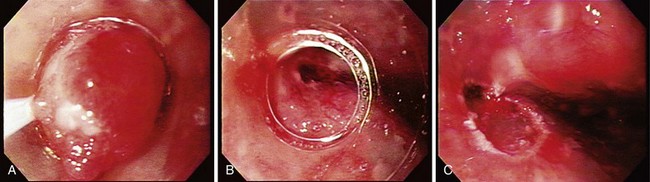
The technique of EMR is performed in a similar fashion. For early esophageal cancers, it is critical that the endoscopist is able to obtain adequate lifting of the lesion to be removed using an injection of a saline-epinephrine solution. Depending on the visibility of the lesion, the borders may need to be marked using a cautery device such as a multipolar coagulator before injection to ensure that the area to be removed can be visualized after injection. The area of carcinoma has usually been previously established by biopsy, and the area is lifted by positioning an injection needle proximal to the lesion. Generally, it is not advisable to inject into the lesion because it is theoretically possible to disseminate cancer cells into the submucosa. The sequence of an injection with adequate lifting of the target lesion is shown in Fig. 27.4.
The sequence of positioning a snare around the lip of a mucosal resection cap is shown in Fig. 27.5. This is often the most difficult portion of the mucosal resection because if the snare is not properly formed, suctioning the tissue into the cap can result in dislodgment of the snare and improper tissue resection. In addition, the snare is easily deformed and can be twisted during the process of forming the loop around the lip of the resection cap resulting in the need for a new crescent snare. When the snare is positioned, it is important that the technician assisting with the snare not move it because even slight disruptions in the position of the snare can cause it to dislodge.
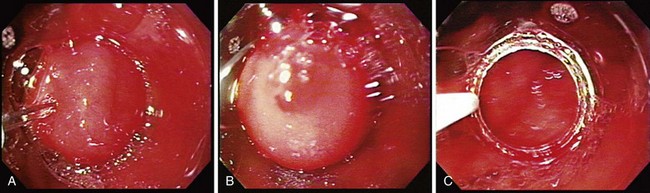
The tissue resection is completed by suctioning the tissue into the cap as shown in Fig. 27.6. To accomplish a wider resection, more tissue must be suctioned into the cap similar to what is done in variceal band ligation. The submucosa is often resected with the mucosa during EMR. When sufficient tissue is suctioned into the cap, the snare is closed, and cautery is applied until the tissue is transected. This process takes several more seconds than with polypectomy because the amount of tissue to be resected is much greater. The average diameter of resected mucosa is about 1 cm when assessed by the pathologist, and the defect left behind by the resection is often about 2 to 3 cm in diameter. The resection and the residual ulcer are also shown in Fig. 27.6. Because tumors are often larger than the size of a single EMR, a second resection can be performed directly adjacent to the first resection; care must be taken not to suction the muscularis propria exposed at the site of the first resection into the cap. Multiple resections in the same area should be attempted only by endoscopists familiar with the mucosal resection technique.
EMR can be used for diagnosis of unusual-appearing lesions within Barrett’s esophagus. In our experience, regions of nodularity or mucosal irregularity have a significantly higher incidence of carcinoma being present. A study of agreement among expert pathologists on distinguishing intramucosal carcinoma versus high-grade dysplasia found only moderate agreement with a κ score of less than .6 even when the pathologists could agree to standard definitions.19 It was thought that limited tissue from pinch biopsies often obscured the pathologist’s ability to determine invasion. Using EMR, our group found that the diagnosis of adenocarcinoma increased by 40% in a group of 25 patients with Barrett’s esophagus because EMR furnishes larger specimens.20 EMR is also an excellent tool for staging esophageal carcinoma as has been shown in Japan.21 It has been established that if the tumor can be found to be m1 or m2 stage (confined to the lamina propria), the tumor can be safely resected with only rare incidence of metastasis. Metastasis was found in 6% of patients who had cancers that penetrated to the muscularis mucosae or superficially into the submucosa. The technique of EMR gives the endoscopist similar tools to evaluate cancer curability as surgeons have had in the past—the ability to obtain histology and pathologic depth of staging.