Chapter 33 Endoscopic Therapy for Gastric Neoplasms
Introduction
The development and clinical application of endoscopic ultrasound (EUS) have enabled GI specialists to evaluate previously inaccessible intramural gastric lesions and to determine the layer of origin. With the ability to determine, with almost pinpoint accuracy, the depth of gastric wall layer involvement and to exclude lymph node metastasis, the endoscopist can safely proceed to endoscopic removal of a gastric neoplasm. Introduction of new techniques such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and thermal ablation techniques such argon plasma coagulation (APC) have made it possible to remove or destroy certain types of gastric neoplasms safely and completely.1–7
A gastric tumor is defined as any mass lesion occurring in the wall of the stomach. Its metastatic potential defines the difference between benign and malignant neoplasms. Epithelial neoplasms include adenomas and carcinomas. Intramural lesions include gastric stromal cell tumors and lymphoma. Box 33.1 shows the classification of gastric neoplasms. The primary emphasis of this chapter is on endoscopic therapy for early gastric cancer (EGC).
Gastric Cancer
Gastric cancer is still the world’s second leading cause of cancer mortality after lung cancer, despite its worldwide decline in incidence and mortality. It has been estimated that 700,000 deaths per year from the disease occurred in 2002 accounting for more than 10% of all cancer deaths.8,9 Adenocarcinoma accounts for approximately 90% of gastric cancers, with the remainder being due to non-Hodgkin’s lymphoma (NHL) and leiomyosarcoma.
Epidemiology
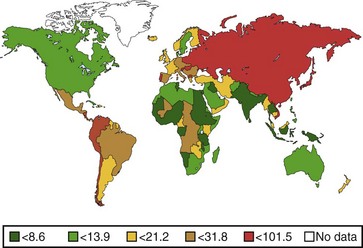
The annual incidence of gastric cancer varies worldwide. There is a difference in the prevalence of gastric cancer between the East and the West (Fig. 33.1).10 In Eastern Asian countries such as Japan and China and in many developing countries, gastric cancer is the most prevalent malignant neoplasm and the leading cause of cancer mortality.11 Fig. 33.2 shows the incidence of gastric cancer in Eastern Asia and the United States.10 There is a high incidence of gastric cancer in the East, the former Soviet Union, some portions of Central and Eastern Europe, and Central America (e.g., Costa Rica) and South America (e.g., Chile). The lowest rates occur in the United States and in some parts of Africa.
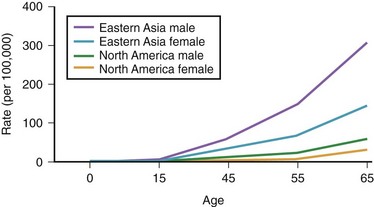
Fig. 33.2 Incidence of gastric cancer in East Asia and the United States according to gender and age.
The International Agency for Research on Cancer (IARC) data for 1996 reported an age-standardized incidence rate of 95.5/105 in Japanese men in Yamagata, Japan, and 7.5/105 in white men in the United States.11 Most of the geographic variation is due to differences in the incidence of gastric cancer not localized in the cardia (noncardia gastric cancer); cancer that is localized in the cardia has a more uniform distribution. Ethnic groups that migrate from a country with a high incidence to a country with a low incidence have an overall risk intermediate between that of their homeland and that of their new country. First-generation migrants tend to retain their high risk, whereas subsequent generations have risk levels approximating that of their host country.12
Both the incidence and the mortality rates for gastric cancer have declined sharply during the last several decades, particularly in the United States and in Western Europe. In the United States, gastric cancer is the 13th most common cancer and the 8th most common cause of cancer death. A steady decline in the incidence of noncardia gastric cancer has occurred since 1930 throughout the world.13 The incidence of adenocarcinoma involving the cardia or the esophagogastric junction, or both, has increased in developed countries such as the United States.14 The reasons for this trend are unknown.
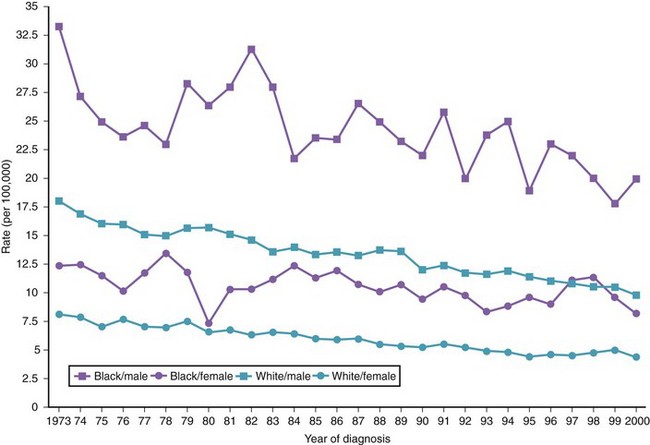
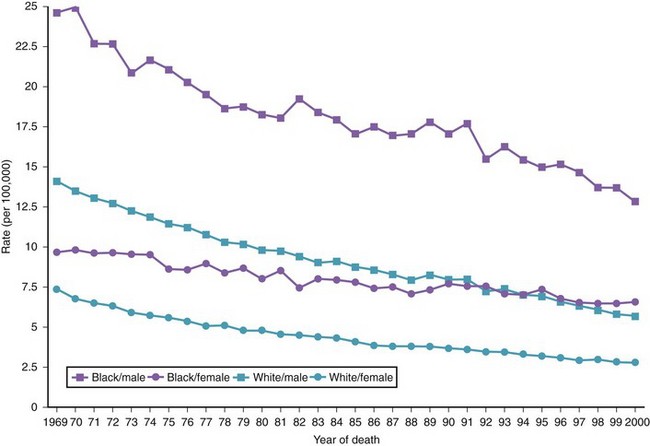
There are variations in the overall incidence and mortality of noncardia gastric cancer between gender and ethnic groups. In the United States, the incidence is greater among Native Americans, Hispanic whites, and African Americans.15 The ethnic distribution for cancer of the gastric cardia is also different, with a preponderance in American whites over African Americans.11 The incidence of gastric cancer increases with age, with most patients 50 to 70 years of age. The incidence of gastric cancer in patients younger than 36 years has increased from 1.8% before 1970 to 4.2% after 1970, with most (62.5%) occurring in Hispanics. Noncardia gastric cancer is more common in men than women by a ratio of approximately 2 : 1. Gastric cancer located in the cardia has an even higher male-to-female ratio of up to 6 : 1 in U.S. whites.16 Figs. 33-3 and 33-4 summarize the trends in the incidence and mortality of gastric cancer in the United States during the years 1975–2006 according to gender and ethnicity.17
Pathogenesis
Gastric cancer has a poor prognosis with a 5-year survival of less than 5%. This poor prognosis is mostly due to the fact that four out of five patients present at an advanced stage.18 Incidental diagnosis of gastric cancer has been reported to increase patient survival.19,20
Japan has the highest incidence of gastric cancer worldwide. However, the establishment of an aggressive mass screening policy has resulted in a remarkably high detection rate of EGC—40% to 66.4% of all gastric cancers diagnosed.21,22 Consequently, the most extensive experience with treatment of EGC has come from Japan.
The Japanese Society of Gastrointestinal Endoscopy defines EGC as cancer confined to the mucosa or submucosa, with or without lymph node metastasis. This designation was based on the observation that this subgroup of patients has an excellent prognosis, with a 5-year survival of greater than 90% after gastrectomy and removal of both the primary and the secondary lymph nodes.1,21 In the West, 10% to 20% of surgical resections for gastric carcinoma are for EGC23; in Japan, more than 50% of surgical specimens are classified as EGC.24,25
Helicobacter pylori
Since the bacterium Helicobacter pylori was first reported in 1983, a wealth of evidence has been gathered concerning its role in the etiology of gastric cancer.26–28 In 1994, the IARC classified H. pylori as carcinogenic to humans. Evidence supporting an etiologic association between H. pylori and gastric cancer can be found in ecologic studies, case-control studies, and prospective cohort studies.27,29–31 Meta-analyses of prospective studies suggest that the risk of gastric cancer is increased twofold to threefold in individuals with chronic H. pylori infection.32,33 One prospective, nested case-control study found a significant association between prior H. pylori infection and gastric adenocarcinoma overall, but there was no association with cancer of the cardia.34 Individuals without H. pylori colonization seem to have a minimal risk of gastric carcinoma.35
H. pylori contributes to the causation of gastric cancer via mechanisms that involve the development and progression of chronic gastritis.36 Infection causes chronic gastritis in almost all infected individuals and accounts for almost all cases of chronic gastritis.37 H. pylori–induced gastritis may progress over time from superficial nonatrophic gastritis to more severe forms, including severe atrophic gastritis with intestinal metaplasia.
Chronic gastritis is present in most cases of gastric cancer and is associated with an increased cancer risk.36 The risk of developing gastric cancer increases with the severity of gastritis, with reported risks greater than 10-fold for severe atrophic antral gastritis.36,38 Intestinal-type gastric cancer seems to be more strongly associated with severe atrophic gastritis, whereas the diffuse type is more common in nonatrophic gastritis.36
There is strong evidence for a role for virulence factors in H. pylori carcinogenesis. The Cag A virulence factor is strongly associated with the risk of adenocarcinoma. The absence of Cag A carries, at most, a low risk of developing diffuse-type adenocarcinoma.39
Peptic Ulcer Disease
H. pylori infection is a common risk factor for gastric ulcer, duodenal ulcer, and gastric cancer. An association between peptic ulcer disease and gastric cancer would be expected. However, studies have found duodenal ulceration to be inversely associated with gastric cancer risk.40
One possible explanation for this apparent paradox was suggested by Parsonnet,41 who argued that H. pylori infection can progress to gastric cancer or duodenal ulcer but seldom to both. Other factors, such as the genetic characteristics of the individual and the organism, may play a significant role in determining which disease would occur. The age at which infection was acquired may also influence the outcome. It has been suggested that infection early in life predisposes to atrophic gastritis and cancer but reduces duodenal ulcer risk owing to decreased acid production associated with the gastritis. If infection is acquired later in life, atrophic gastritis is less likely, and gastric cancer risk is decreased.41 More recent evidence supports a moderate association between gastric ulcer and noncardia gastric cancer.42,43
Dietary Factors
Several observational studies have shown a protective association with fresh fruits and vegetables, independent of other dietary factors. The association has been less impressive in limited cohort studies.44 Possible protective nutrients include vitamin E, carotenoids, selenium, and vitamin C.44 The evidence is stronger for vitamin C. In a case-control study, a high intake of vitamin C decreased the risk of gastric cancer by half compared with low vitamin C intake.45 However, a large 5-year intervention trial in China involving adults did not show any change in the risk of gastric cancer in individuals receiving supplemental vitamin C.46
High salt consumption has been fairly consistently associated with an increased risk of gastric cancer in ecologic and case-control studies.47–49 However, good quantitative data linking salt consumption with gastric cancer are still lacking.
N-nitroso compounds have been shown to be carcinogenic in animal studies.50 In the human stomach, these compounds may be formed from dietary nitrite or nitrate, leading to the hypothesis that a diet high in nitrite or nitrate predisposes to gastric cancer. Case-control studies examining dietary intake of nitrate and the risk of gastric cancer have consistently found a negative association. Because the major sources of nitrate and nitrite are vegetables and preserved meats, nitrate intake was probably an index of vegetable intake, and the negative association is not surprising.44 Case-control studies have reported a weak, statistically insignificant increased risk of gastric cancer (relative risk 1.12 to 1.28) for high versus low nitrite intake.51–54 It is difficult to isolate the effect of nitrate consumption in gastric cancer because of the fact that diets high in nitrite may also be high in antioxidants that are frequently found in vegetables and in fruits.54
It has been postulated that virulent strains of H. pylori release reactive oxygen metabolites that could destroy neighboring glandular cells leading to gastric glandular atrophy, hastened by other factors such as high salt intake but retarded by antioxidants such as vitamin C.55 The invention of refrigeration, the availability of fresh food, and the accompanying decreased consumption of preserved foods may have contributed to a decline in gastric cancer incidence in the second half of the 20th century.
Socioeconomic Status
Worldwide, a low socioeconomic status has been consistently shown to be associated with an increased risk of gastric cancer.16 The higher prevalence of gastric cancer among individuals with lower socioeconomic status is matched by a similar high prevalence of H. pylori infection in these individuals.56,57 An increased incidence of cancer of the cardia has been observed predominantly in individuals with higher socioeconomic status.14
Gastric Surgery
An increased risk of gastric cancer after gastric surgery has been reported, particularly after 15 or more years.58,59 The association is strongest for gastrectomy performed for gastric ulcer but less persuasive for either vagotomy or gastrectomy performed for duodenal ulcer. This association does not extend to cancer of the gastric cardia.40
Gastric Polyps
Single hyperplastic polyps are often found in the background of chronic gastritis, and they are mainly seen in the body of the stomach. At most, 0.3% of hyperplastic gastric polyps may contain a focus of adenocarcinoma. However, approximately 3% of multiple hyperplastic polyps are associated with a synchronous carcinoma elsewhere in the stomach.60 The presence of multiple hyperplastic polyps is considered a precancerous condition.
Fundic gland polyps do not have malignant potential when they occur sporadically or in association with proton pump inhibitor therapy. Hundreds of fundic gland polyps may be present in association with polyposis coli. Dysplasia has been reported in a few of these patients.61 Gastric adenocarcinoma associated with fundic gland polyposis in familial polyposis coli has been reported.62
Adenomatous polyps occur in the setting of intestinal metaplasia and associated chronic gastritis. Endoscopic features that increase the risk of malignant transformation include large size and a red, erosive surface color. A carcinoma may be contained in 40% of pyloric gland adenomas and generally 10% of tubular adenomas.60,63 Approximately 11% of patients with adenomatous polyps have a synchronous or metachronous gastric adenocarcinoma.63
Genetic Factors
Genetic predisposition may play a role in the development of gastric cancer, with a twofold to fourfold increased risk in first-degree relatives. Similarly, patients who have hereditary lesions, such as Lynch syndrome II, have an increased risk of gastric cancer.64,65
Miscellaneous Factors
A higher risk for gastric cancer has been reported in patients with Ménétrier’s disease, ataxia telangiectasia, and blood type group A.66–68 Pernicious anemia and its association with autoimmune chronic gastritis increases the risk of developing gastric cancer by twofold to threefold, according to population-based studies.69,70 Regular endoscopic surveillance in young patients with pernicious anemia has been advocated.71
Smoking may increase the incidence of premalignant gastric lesions and dysplasia; however a dose-response relationship has not been clearly shown.72–74 No clear relationship between alcohol consumption and gastric cancer has been shown.29,72,74
Molecular Abnormalities
Abnormalities at the molecular level have been described in patients who develop gastric cancer. These include allelic deletions of the atrial premature complex (APC) gene, the migrating motor complex (MCC) gene, and the tumor-suppressor gene TP53. Allelic deletions of TP53 can be found in 64% of gastric cancers. These deletions are common late events in gastric cancer.75 In addition, mutations of the APC gene and loss of heterozygosity on chromosomes 1q, 5q, and 17p have been reported in gastric carcinoma.76
Loss of E-cadherin–dependent cell-cell adhesion secondary to mutation of beta-catenin has been found in gastric cancer.77 Reduced expression of E-cadherin has been found to be significantly associated with recurrence of cancer and decreased survival.78 Microsatellite instability has also been found in gastric carcinoma.79 Higher expression of Sialyl-Tn has been associated with a poor prognosis in patients with gastric cancer.80 Patients with overexpression of c-erbB protein have been found to have a poorer prognosis than patients without c-erbB expression in poorly differentiated gastric adenocarcinoma.81 Germline truncating mutations in the E-cadherin gene (CDH1) have been found in several families with hereditary diffuse gastric cancer.82,83
Clinical Features
Advanced Gastric Cancer
Most patients with gastric adenocarcinoma present with advanced disease. Accompanying symptoms, such as weight loss, vomiting, anorexia, early satiety, abdominal pain, and anemia, may mimic peptic ulcer disease and other GI conditions. Typically, patients have been symptomatic for less than 12 months, and 40% of patients have been symptomatic for less than 3 months. Signs and symptoms are due to cancer invasion beyond the muscularis propria by direct extension or by distant metastasis. Liver and lungs are the most common sites of gastric cancer metastases (40%); bone and peritoneum are less frequent sites (10%). Occasionally, patients may present with a paraneoplastic syndrome, such as Trousseau’s syndrome (thrombosis), acanthosis nigricans (pigmented skin lesion, classically of the axilla), or dermatomyositis.84,85
Pathology
Most primary gastric cancers are adenocarcinoma, with an occasional squamous or adenosquamous carcinoma. Other rare gastric cancers include parietal cell carcinoma, choriocarcinoma, and rhabdoid tumor. Cancer metastasizing to the stomach is uncommon, with lung cancer being the most frequent primary tumor. A few cases of gastric carcinosarcoma and spindle cell carcinoma have been reported.86
Histologically, gastric adenocarcinoma can be divided into two categories87: (1) intestinal type, a well-differentiated neoplastic lesion that forms glandlike structures that ulcerate frequently, and (2) diffuse type, characterized by infiltration and thickening of the gastric wall without the formation of a discrete mass. Approximately 16% of gastric cancers cannot be categorized or are of the mixed type.86 The decline in incidence of gastric cancer has been predominantly in the distal stomach intestinal type, whereas a steady increase in the incidence of the proximal stomach diffuse type has been observed in the United States and in Europe.88,89 There has been inconsistent classification of gastric cardia and distal esophageal cancer in the literature because many, if not most, of these represent distal spread from specialized intestinal metaplasia of the distal esophagus or the esophagogastric junction.
Staging
Initial staging of gastric cancer should include spiral computed tomography (CT) of the abdomen to determine the presence or absence of metastatic disease. In the absence of metastasis, EUS is helpful for assessing resectability. Determination of surgical resectability for cure using EUS has been found to be 91% accurate.91
EGC is defined by the Japanese Society of Gastrointestinal Endoscopy as gastric cancer confined to the mucosa or submucosa, with or without lymph node metastasis. Patients with EGC have an excellent 5-year survival of greater than 90%.1,92 The risk of lymph node metastasis is 3% for intramucosal lesions (T1m) and 20% if there is submucosal involvement.93 Other independent risk factors for lymph node metastasis in EGC include lymphovascular invasion on histopathology, histologic ulceration, and tumor diameter more than 3 cm.94 For T1m tumors less than 3 cm without evidence of lymphovascular invasion or histologic ulceration, the risk of lymph node metastasis is only 0.36%. Gotoda and colleagues95 assessed the risk of lymph node metastasis of intramucosal cancer. None of the 1230 well-differentiated intramucosal cancers that were less than 30 mm in diameter regardless of ulceration findings were associated with metastases. None of the 929 lesions without ulceration were associated with nodal metastases regardless of tumor size. Similar to findings for intramucosal cancers, for submucosal lesions, there was a significant correlation between tumor size larger than 30 mm and lymphatic-vascular involvement with an increased risk of lymph node metastasis. None of the 145 differentiated adenocarcinomas less than 30 mm in diameter without lymphatic or venous permeation were associated with lymph node metastasis, provided that the lesion had invaded less than 500 µm into the submucosa.95
Treatment
Gastroscopy
A morphologic staging classification based on the macroscopic endoscopic appearance of EGC has been described by Japanese investigators, as follows96:
Types I, IIa, and IIc are considered to be endoscopically resectable. Certain adjuncts to morphologic staging also help to predict that a lesion is amendable to minimally invasive (endoscopic) therapy and is likely to have the same outcome as surgery: lesions less than 1 cm in size, absence of a scar or ulcer histologically and endoscopically (0.2% lymph metastasis), and a well-differentiated histology (<1% lymph metastasis).1,92
Narrow Band Imaging and Magnification Endoscopy
Diagnosis of gastric neoplasia using magnification endoscopy has been attempted in the past decades.97 However, compared with the colonic surface, standardized systemic classification of the magnified gastric surface has not been established owing to diverse histology of gastric carcinomas; the existence of three different types of proper gastric mucosa; and various changes on mucosal structures by atrophy, chronic inflammation, metaplasia, and erosions. The narrow band imaging system provides very clear images of fine superficial structure and microvasculature of the gastric mucosa and has improved the endoscopic diagnosis of superficial gastric cancers. Nakayoshi and coworkers98,99 reported that significant correlation between histopathology and microvascular pattern obtained with narrow band imaging magnification enables the sensitive diagnosis of superficial depressed gastric cancer, and the modality can assist in determining feasibility of EMR of gastric cancer.
Nakayoshi and coworkers98 measured the correlation between the magnified images obtained with narrow band imaging and the histologic findings, especially with regard to the microvascular pattern; 165 cases of superficial depressed gastric carcinoma (109 well-differentiated adenocarcinomas and 56 poorly differentiated adenocarcinomas) were evaluated. Microvascular patterns on the mucosal surface were classified into two patterns: fine network pattern and corkscrew pattern. The fine network pattern appears as a mesh, and abundant microvessels are well connected with one another. In contrast, the corkscrew pattern has isolated and tortuous microvessels and looks like a corkscrew. A fine network pattern was recognized in 72 cases (66.1%) of 109 well-differentiated adenocarcinomas (intestinal type). The corkscrew pattern was observed in 48 cases (85.7%) of 56 poorly differentiated adenocarcinomas (diffuse type). For the magnifying endoscopic diagnosis of superficial depressed gastric carcinoma, both the existence of irregular microvessels and the disappearance of fine superficial structure are essential.
Endoscopic Ultrasound and Endoscopic Ultrasound–Guided Fine Needle Aspiration Cytology
EUS is now established as the best local staging modality for esophageal, gastric, and rectal cancer with a T stage accuracy of about 90%.100,101 Kelly and associates91 reported that T staging by EUS was more accurate for gastric cancer than for esophageal cancer, although there was no difference for nodal staging. EUS has been shown to be the most accurate imaging modality for staging EGC,101,102 with an accuracy of greater than 90% for assessing the depth of tumor invasion.103,104
Long-term disease-free survival in GI malignancies is a function of lymphatic and distant tumor spread. The linear-array echoendoscope has enabled the endoscopist to place a needle precisely under real-time EUS guidance and obtain tissue samples both from the primary tumor and from transluminal lesions such as lymph nodes and liver metastasis. Other imaging modalities, such as CT scan, are still necessary to exclude more distant metastases. The overall performance of EUS with fine needle aspiration (FNA) cytology for benign versus malignant lymph node involvement has been found to have a sensitivity of 92% (range 84% to 97%), specificity of 93% (range 75% to 100%), and accuracy of 92% (range 82% to 98%).105 Disadvantages of dedicated echoendoscopes include inability to traverse critical stenoses and technical difficulty of evaluating superficial lesions in some areas of the stomach such as the cardia.
High-Frequency Ultrasound Probes
The miniature probe can also be used to evaluate a lesion after saline injection, to confirm complete separation of the lesion from the underlying muscularis propria layer of the gastric wall before EMR.106 Inability to separate the lesion from the muscularis propria after saline injection is a predictor of unresectability and obviates the need for additional imaging to determine endoscopic resectability.
High-Frequency Ultrasound Staging of Early Gastric Cancer
A classification system for EGC has been developed based on depth of tumor penetration into the mucosa (m) and submucosa (sm), as defined by using HFUS probes. The superficial cancers are divided further into the upper, middle, and lower third of the layer involvement as m1, m2, m3, sm1, sm2, and sm3.103
An overall T staging accuracy of about 80% has been reported using HFUS in patients with EGC.103,107,108 T staging accuracy is greater for Tm1 (92.1%) than for Tsm1 (62.8%) or T2 muscularis propria (42.9%).109 The curative effect of EMR is excellent for intramucosal cancers (m1, m2, and probably m3) but not for submucosal cancers (sm1 to sm3).14 The ability to distinguish between m3 and sm1 lesions is crucial. Using a 20-MHz frequency probe, Yanai and colleagues110 were able to distinguish EGC involving the mucosa from EGC involving the submucosa with an accuracy of 72.3%.
The usefulness of HFUS was evaluated in 33 patients referred for endoscopic management of superficial or submucosal neoplastic lesions.111 The depth of invasion was accurately predicted by HFUS before EMR in 25 of 26 patients (96%). Of the nine gastric lesions removed, eight had HFUS before EMR. There was 100% agreement between the depth of invasion determined by HFUS and pathologic staging. Almost half of the resected lesions were gastroduodenal, including gastric adenocarcinoma, gastric and duodenal adenoma, gastric and duodenal carcinoid, pancreatic rest, fibrovascular submucosal polyp, ampullary adenoma, and duodenal lipoma. The diagnostic accuracy of HFUS probes for small gastric lesions (<4 cm) has been found by some researchers to be comparable to dedicated (standard) echoendoscopes.112
Description of Techniques
Endoscopic Mucosal Resection
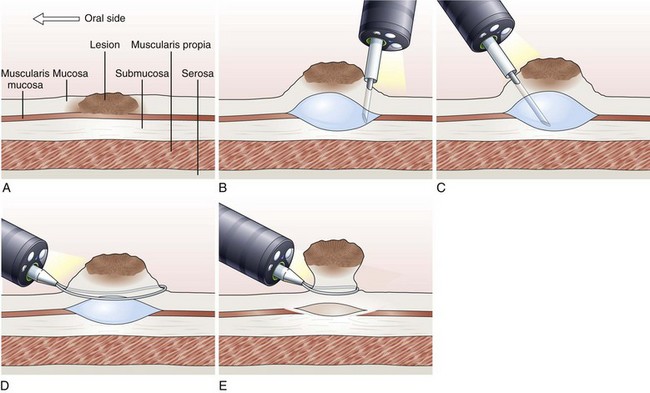
EMR involves the lifting of a lesion from the deep muscle layer of the gut wall, either by injection or by suction of the lesion into a cap fitted to the tip of the endoscope, followed by snare removal of the lesion (Fig. 33.5). Complete removal of the lesion en bloc during a single therapeutic procedure is ideal, although large lesions may require piecemeal resection. The availability of the resected specimen for pathologic examination is a distinct advantage of EMR over ablation therapies. Because EUS and HFUS cannot reliably distinguish between tumor infiltration and inflammation that may be associated with a malignant lesion, EMR provides both curative benefit and final staging confirmation.
The following EMR techniques have been described113–115:
Inject and Cut Technique
In the inject and cut technique, the lesion is lifted from the underlying muscularis propria by injecting a solution submucosally to produce a bleb beneath the lesion. The lesion is captured and resected by using an electrosurgical snare (Fig. 33.6). The required volume of submucosal injection varies according to the size of the lesion, provided that the bleb is sufficient to ensure a good lift of the entire lesion so that it can be safely captured and resected.
Various solutions are available for injection. A hypertonic saline and epinephrine solution is widely used in the hopes of decreasing the risk of bleeding116; other solutions have been used to obtain a more durable lift of the lesion.117 The use of hypertonic solutions except for glycerol has been questioned more recently because of concern about possible tissue damage. A study by Fujishiro and associates118 concluded that a combination of hyaluronate and glycerol is the most favorable submucosal injection solution, taking into consideration tissue damage and lesion-lifting ability. Also, various snares and currents (e.g., blended, ERBE, Tübingen, Germany) are preferred by different endoscopists. To date, no randomized trials have been conducted to compare the safety and effectiveness of different types of snares and injectants.
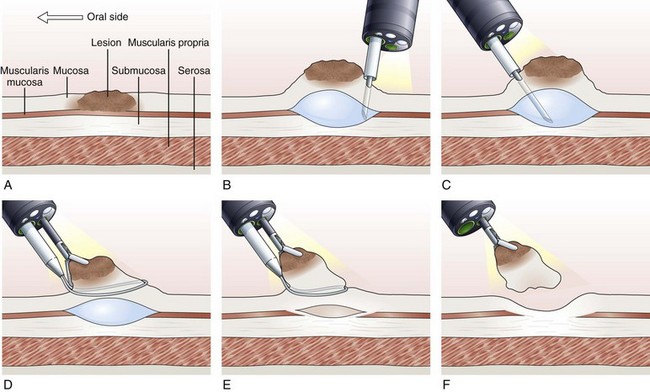
Inject, Lift, and Cut Technique (Strip Biopsy)
With the inject, lift, and cut technique, the submucosal injection is performed in the standard manner, as already described. A snare and grasping forceps are passed through the operating channels of a dual-channel endoscope. First, the grasping forceps is captured by the open snare, and the snare is closed over the forceps. Working as a unit, the forceps is used to grasp the lesion, the snare is opened, and the lesion is pulled through the open snare. The snare is closed over the lesion, and resection of the lesion is performed (Fig. 33.7).119–121 This technique is more cumbersome than the inject and cut technique and requires a dual-channel endoscope and two assistants to perform EMR.
Endoscopic Mucosal Resection with Ligation
In the EMR with ligation technique, the lesion is removed with or without previous submucosal injection (Fig. 33.8).122–124 More recently, a conventional band ligation device (MBL; Wilson-Cook Medical, Inc, Winston-Salem, NC) was modified by widening the threading channel of the cranking device from 2 to 3.2 mm.125 This device allows for the insertion of a 7-Fr catheter through the threading channel of the cranking device into the 3.7-mm working channel of the endoscope. Band ligation can be performed with the polypectomy snare still within the working channel without any increased friction during winding of the thread; this enables sequential banding and snare resection of esophageal mucosa without the need to change the endoscope.125
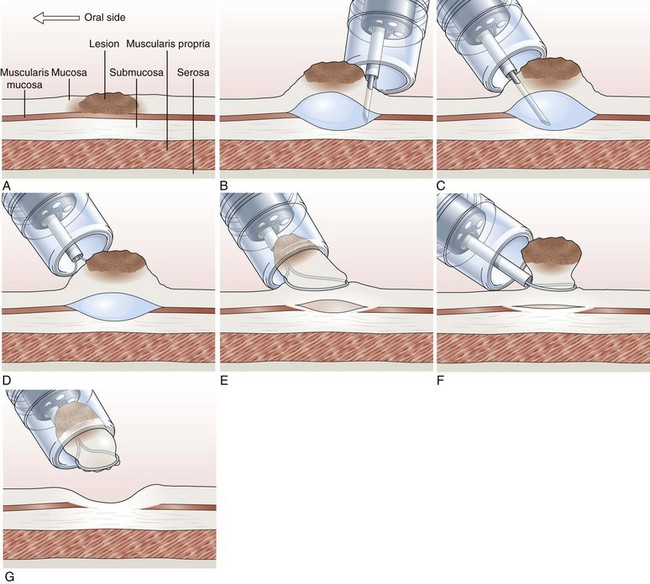
Cap-Assisted Endoscopic Mucosal Resection
In cap-assisted EMR, a specially designed transparent plastic cap is used.126,127 The plastic cap is fitted over the tip of the endoscope; various cap sizes are available. Submucosal injection of the lesion is performed in the standard manner. A crescent-shaped snare is prelooped into the groove of the rim of the specialized cap by gently suctioning normal mucosa into the cap and opening the snare to allow it to rest along the inside groove of the rim of the cap (SD-221L-25 or SD-7P-1; Olympus America, Inc). After prelooping the snare, the suction is turned off to release the normal mucosa. The cap is now used to suction the lesion while maintaining a constant medium to high vacuum. When the lesion is trapped completely inside the cap, the snare is closed over the lesion. After the lesion is tightly strangulated by the snare, the suction is turned off, and the lesion with the snare around it is allowed to leave the cap. Resection of the lesion is then performed with application of current. Gentle suction of the specimen into the cap allows safe and complete recovery of the specimen (Fig. 33.9). This technique is particularly useful for upper GI lesions.127–129 Matsuzaki and colleagues128 reported the use of a soft 18-mm diameter cap for en bloc resection of larger gastric lesions up to 1.4 times the size that can be removed with a standard cap.
For larger lesions greater than 3 cm, some endoscopists have used a more viscous material, sodium hyaluronate, for submucosal injection. A small-caliber tip transparent hood that accommodates a needle-knife or an insulated thermal knife with a ceramic cap is used to cut around the lesion on its entire circumference. The final step is the complete removal of the lesion using a large snare.130,131
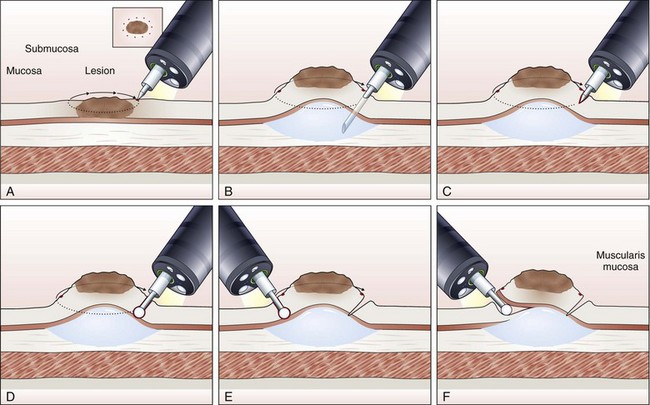
Endoscopic Submucosal Dissection
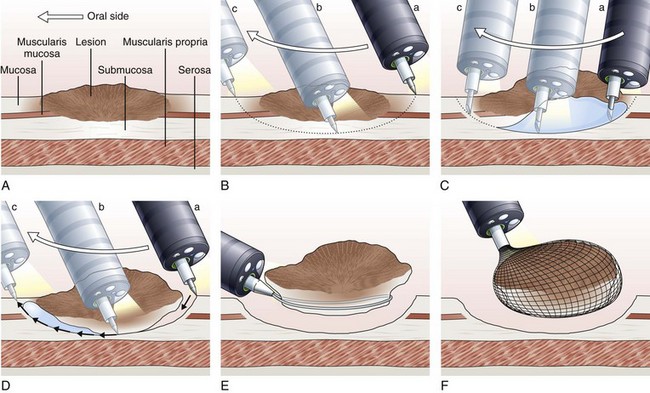
The concept and technique of ESD are markedly different from conventional EMR. The procedure begins by making circumferential markings all around the lesion at about a 5-mm distance, at 2-mm intervals. Injection of sodium hyaluronate or another solution to create a long-lasting submucosal fluid cushion between the lesion and the muscle layer is performed to prevent perforation during ESD (Fig. 33.10).117
Sodium hyaluronate is harmless to the injected tissue because of its isotonicity, but it needs to be diluted when used for ESD because of its high viscosity. Among different mixtures tested, a mixture of high-molecular-weight sodium hyaluronate with a sugar solution, with or without glycerin, seems to be the most cost-effective.132 Adding indigo carmine dye (0.004%) to the mixture is optional. To minimize resistance to injection, a high-flow injection needle with a large inner lumen should be used. The solution is first injected just outside the marking spots, where a circumferential incision to isolate the lesion to be resected from the normal mucosa is made using the electrosurgical knives selected for the procedure. For the insulated-tip (IT) knife, a small initial incision is made with a standard needle-knife to allow for its insertion into the submucosal layer. Several electrosurgical knives have been developed for ESD.
Needle-Knife
First used by Hirao and colleagues116 in 1988, the needle-knife (KD-10Q-1; Olympus) has a fine tip and a small contact area, which allows a sharp incision. The cutting power required for mucosal incision (150 W) is so high that perforation is likely to occur unless the operator is highly skilled.
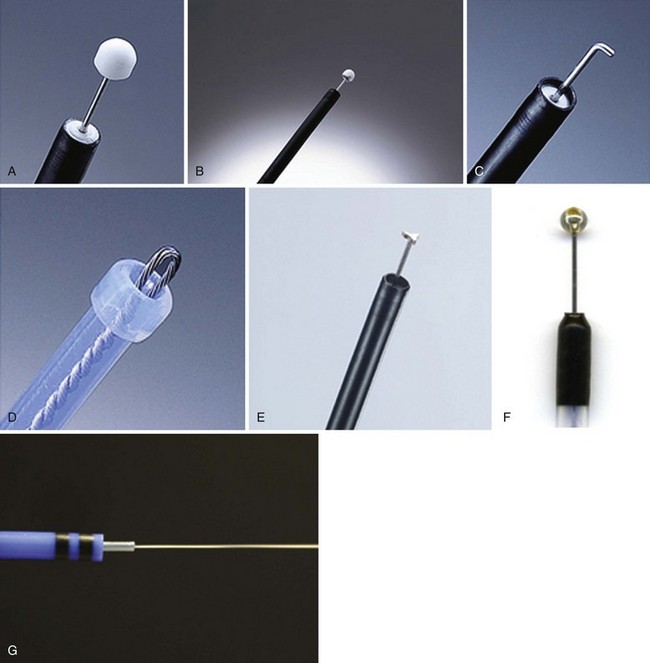
Insulated-Tip Knife
The IT knife (KD-610L; Olympus; Fig. 33.11A) is a needle-knife that is equipped with a ceramic ball at the top of the incising needle. The tip is blunted and insulated to prevent perforation; the knife was developed by Hosokawa and Yoshida133 for safe performance of ESD. The IT knife is most frequently used for ESD of the stomach; special care should be taken when operating in the colon. The second-generation IT knife (ITKnife2; KD-611L; Olympus; Fig. 33.11B) has an additional electrode at the back of its insulating tip that makes cutting easier, especially in the lateral direction.
Hook Knife
The hook knife (KD-620LR; Olympus; Fig. 33.11C) is a needle-knife with 1 mm of the tip bent to form a right angle. Submucosal tissue can be hooked and pulled before incision, which improves safety. Safety can be enhanced further by the concomitant use of a transparent hood. This knife has a rotating function so that operators can select any direction for hooking. Because of its high maneuverability, the hook knife is a useful endo-knife for esophageal ESD.134
Flex Knife
The flex knife (KD-630L; Olympus; Fig. 33.11D) has a rounded tip with a twisted wire similar to a snare, with a length that can be adjusted according to circumstances. The outer sheath of the knife is soft and flexible with a thick tip that functions as a stopper. Because of all these characteristics, perforation is less likely to occur with this needle, which can be used independently on the location of the lesion.135 A transparent hood for better visualization of the operating field is usually used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree