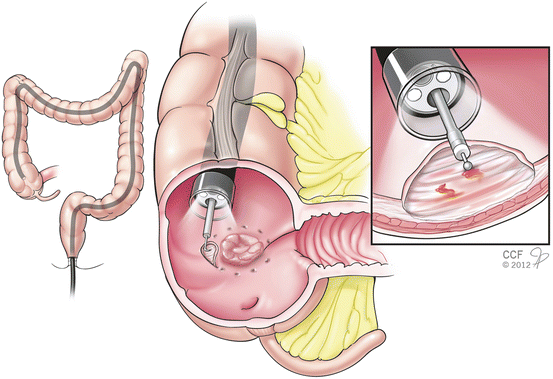
Fig. 1.1
Endoscope system (colonoscope)

Fig. 1.2
Biopsy port
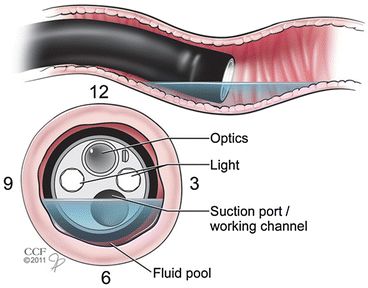
Fig. 1.3
Manipulation of the “biopsy port” to the 6 o’clock position (Reprinted with permission, Cleveland Clinic, Center for Medical Art & Photography © 2011–2014. All Rights Reserved)
The need for further improvement in endoscopic techniques led to the development of a two-channel video colonoscope in 1993 [5]. Since this design has two instrument channels, both a grasping forceps and a snare can be inserted into the bowel lumen at the same time (Fig. 1.4). This enables lesions to be pulled into the center of the lumen and creates traction for electrocoagulation by the snare. The two-channel configuration maximizes versatility by permitting two instruments to be used simultaneously. Suction function can also be used from one or both channels concurrently. In dual-channel scopes, the two-channel construction incorporates one larger (3.2–3.8 mm) and one smaller (2.8 mm) diameter channels. One early study in 1996 demonstrated the use of a two-channel video colonoscope in the treatment of small carcinoid tumors of the rectum [6]. They reported that the complete resection rate for rectal carcinoids was significantly higher with a two-channel video colonoscope (90 %) than with a conventional one-channel scope (29 %), with neither bleeding nor perforation during or after treatment. However, many endoscopists believe that the operation of two instruments through the flexible endoscope is extremely challenging, especially when the instruments are intended to move in opposing directions. Therefore, endoscope manufacturers are currently working to develop articulating arms at the end of the flexible colonoscope which would function independently from each other and from the motions of the scope itself.


Fig. 1.4
Dual channel scope, with two side-to-side instrument channels
Success in colonoscopy depends on the ability to meticulously examine the mucosa behind folds and corners. Technologies have been developed to improve the ability to expose the mucosa on the proximal side of a colonic fold and beyond the corners. Among these advances are the wide-angle colonoscopy and the Third Eye® Retroscope® (Avantis Medical, Sunnyvale, California, USA). A wide-angle colonoscope produces a much wider field of visualization (170°) and has produced operator-dependent improvements in efficiency with faster withdrawal time. However, one randomized prospective trial did not report an overall improvement in rate of adenoma detection [7]. With the Third Eye® Retroscope®, a camera is passed down the instrument channel and provides a continuous retroflexed view on a second monitor. The endoscopist watches both the forward view from the colonoscope and the retrograde view simultaneously on side-by-side monitors. In a recent multicenter randomized controlled study investigating this technology, the polyp miss rate was lower when colonoscopy was first performed with the Third Eye® Retroscope® (18.4 %) compared with standard colonoscopy (31.4 %) [8, 9]. However, withdrawal time was significantly longer with the Third Eye® Retroscope®. This is likely in part because of the time required to remove and reintroduce supplementary instruments when a polyp is discovered, and also from the challenge of watching two screens simultaneously. Currently, single-port high-definition videoscopes are the most commonly utilized colonoscopes around the world (Fig. 1.1).
Types of Snares
A polypectomy snare consists of a thin wire loop attached by a long connector that is enclosed within a 7-French plastic sheath. The plastic sheath holding the snare is passed through the “biopsy/suction” channel of the scope. The wire loop is opened and closed using the control handle (Fig. 1.5). This is controlled either by the endoscopy assistant or by the endoscopist. Some endoscopists prefer to hold the snare handle at the time of the polypectomy to feel tissue resistance and to control the speed of tissue transection. The snare handle connects to a generator via an electrosurgical cautery cord. Most snares are monopolar and require a grounding pad to complete the electrical circuit.
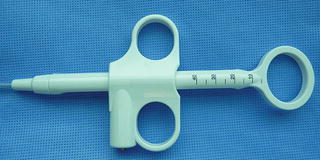

Fig. 1.5
The control handle used to open and close a snare
There are different types of polypectomy snares in regard to loop diameter, shape, design, and filament diameter. The wire loop is typically produced from braided stainless steel wire, which combines strength, memory of shape, and electrical conductance. More rigid monofilament snares allow faster transection over coagulation. The shape of the wire loop is usually oval, elliptical, and hexagonal (Fig. 1.6). Single-use snares are designed for easy insertion into the scope channel and provide more tactile feel and may reduce the risk of cutting too quickly. The soft snares feature a softer, more pliable wire so less force may be needed to open and close the loop. Rigid spiral snares are uniquely designed to minimize mucosal slippage when removing a flat lesion. The oval snares feature a thicker diameter wire designed to deliver a slower and more controlled cut. There are three commonly used oval snare sizes: small (1.5 × 3 cm), standard (2.5 × 5 cm), and “jumbo” (3 × 6 cm).
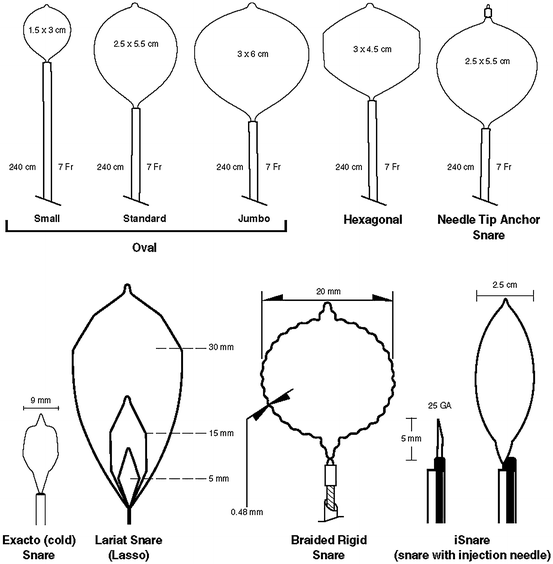

Fig. 1.6
Different shapes and sizes of snares
A needle tip snare provides secure anchoring of the snare tip to the mucosa, which prevents slippage of the snare at the initiation of snare closure. The Exacto® cold snare (US Endoscopy, Mentor, Ohio, USA) is a small (9 mm) snare that is used without electrocautery in circumstances of precise polyp excision (Fig. 1.6). The smaller diameter and shape of the snare allow for increased control of snare placement and resection. The Lariat® Lasso snare is a new polypectomy tool where three different sizes and shapes are available within the same instrument (Fig. 1.6). The configuration memory is a feature of the wire that allows the snare to be changed from oval (30 mm) to hexagonal (15 mm) to rhomboid (5 mm). The author’s own experience with this new multi-shaped snare has been extremely satisfactory. Braided rigid snares deliver minimal slippage on the mucosa and are very beneficial for flat lesions (Fig. 1.6). Lastly, the iSnare® (US Endoscopy, Mentor Ohio, USA) is a snare that comes with a 25 G injection needle within the same sheath (Fig. 1.6). This adds efficiency during polypectomy procedures by eliminating device exchanges between needle injections and snare excision.
It is important to stock small, standard, and large-loop snares and maintain an appropriate inventory of “nonslip” snares, so that the correct snare is always available when needed. Additionally, one must not underestimate the importance of educating endoscopic assistants about the different types and specifications of snares; this will increase the chance of success during advanced endoscopic procedures.
Tools Used in Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection
One technique of endoscopic mucosal resection (EMR) involves the use of a variceal banding technology where a lesion is suctioned into a distal banding cap, and a flat lesion is turned into a pedunculated lesion. The narrow neck is then snared, and the lesion is removed easily. However, endoscopic submucosal dissection (ESD) involves a more advanced technique where submucosal injection and elevation of tissue planes are first achieved, followed by submucosal dissection using various types of needle knives. The advantage of ESD is that it allows an en bloc resection of an intestinal lesion, regardless of the size. This technique was first popularized in Japan for the treatment of early esophageal and gastric cancers [10]. The ESD method is widely used in the field of the upper gastrointestinal tract, especially in the stomach, because an en bloc resection not only offers postoperative organ preservation but exact histopathological diagnoses as well. In Japan, ESD is even performed for the treatment of early gastric carcinoma and superficial esophageal carcinoma.
The use of ESD for colorectal lesions has not yet been established as a standard therapeutic method; however, the use of ESD for colorectal lesions has been successful and studies are ongoing. Many types of endoscopic knives have been introduced and are available for use in colorectal ESD. Currently in the USA, there is no extensive experience with ESD. Our institution recently presented our early experience with ESD at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) annual meeting in 2013 [11]. Since this presentation, we have increased our experience in colonic ESD. A total of 25 patients were referred to us for oncologic colorectal resection for large colorectal lesions. All studied patients were offered initial ESD in the operating room with possible bowel resection if ESD could not be successfully completed. A pediatric colonoscope (Olympus America Inc, Center Valley, PA) was used, and a transparent distal disposable cap was attached to the tip of the endoscope (Fig. 1.7). The lesion was first critically visualized either by dye injection, narrow-band imaging, or direct view. After this step, circumferential marking of the lesion with electrocoagulation was performed (Fig. 1.8). This was followed by submucosal injection using a mixture of saline, 2.5 % Hypromellose (HUB Pharmaceuticals, LLC, Rancho Cucamonga, CA) (Fig. 1.9) and indigo carmine solution (Fig. 1.10). This raises the submucosal plane and allows the procedure to be performed safely. The next step was mucosal incision with the dual knife, followed by submucosal dissection. The submucosal dissection was carried out by the alternating use of the DualKnifeTM, HookKnifeTM, and Coagrasper TM (Olympus America Inc., Center Valley, PA) (Figs. 1.11, 1.12, and 1.13). The disposable distal cap facilitated the dissection in the correct submucosal plane. Once the entire lesion was dissected free, en bloc tissue retrieval was achieved and finally hemostasis was completed. In our series, the median age of the patients was 63 (range 50–88), median ASA score was 4 (2–5), and median body mass index (BMI) was 31 kg/m2 (18–46). Lesions were located in the cecum (40 %), splenic flexure (20 %), sigmoid colon (20 %), transverse colon (10 %), and rectum (10 %). ESD was possible in 20 of 25 lesions (80 %). Median operating time was 114 min (62–196). In five patients, ESD could not be technically performed due to non-lifting of the lesion, and either laparoscopic resection or endoscopic full thickness excision with laparoscopic repair of the defect was performed. There was no perforation or bleeding after ESD. The median length of hospital stay was 1 day (0–5). Follow-up colonoscopy was performed at 3 months, and no recurrence was encountered.