Fig. 6.1
Fluoroscopic image of a catheter advanced into a malignant colonic stricture
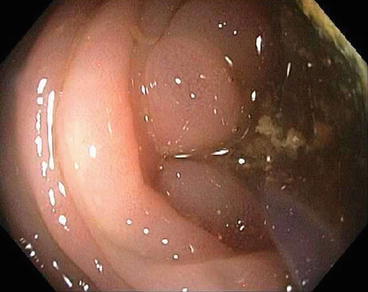
Fig. 6.2
Endoscopic image of a catheter crossing a malignant stricture
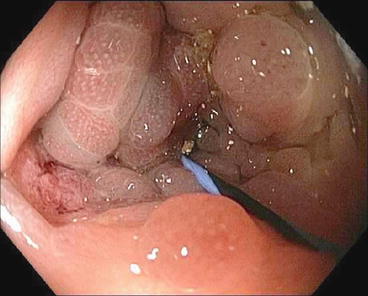
Fig. 6.3
Endoscopic image of a wire placed crossing the stricture
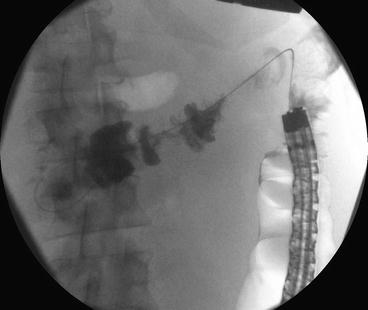
Fig. 6.4
Fluoroscopic image of a balloon catheter inflated to delineate length of the malignant stricture
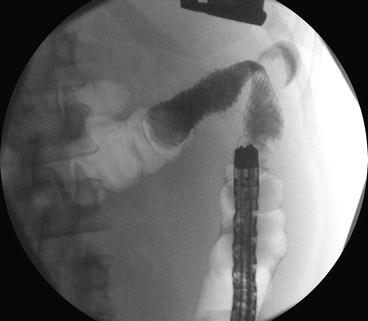
Fig. 6.5
Fluoroscopic image of a self-expanding metal stent crossing the malignant stricture
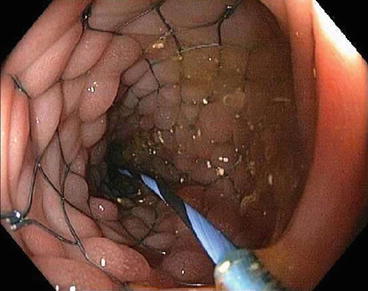
Fig. 6.6
Endoscopic image of a self-expanding metal stent crossing the malignant stricture
Non-Through-the-Scope Stent Placement
This method is preferred by some in distal left-sided lesions in order to assess the entire lesion under endoscopic guidance [9]. A smaller-caliber endoscope (<10 mm) is passed through the lesion to allow placement of a stiff guidewire as high as possible beyond the lesion [8, 9]. The endoscope is then withdrawn while the position and length of the lesion are evaluated [9]. After the predeployed stent is passed across the lesion, the endoscope is reinserted in order to monitor the exact position of the distal end of the stent during deployment [9].
Tips and Tricks
It has been our experience that the TTS endoscopic technique where placement is through an endoscope under direct visualization with or without fluoroscopy is more successful than radiological placement alone or over the wire technique. This is especially true when dealing with a very angulated rectosigmoid anatomy or when the obstruction is proximal to the rectosigmoid.
Passage of a guidewire is a limiting step to successful SEMS placement especially in patients with complete obstruction [8]. Helpful tips include but are not limited to the following: using hydrophilic biliary guidewires (e.g., Glidewire; Boston Scientific, Natick, Mass); having a triple-lumen biliary occlusion (stone retrieval) catheter with the balloon inflated to a large diameter of 15 mm and positioned just distal to or impacted at the tumor site then injecting contrast in order to measure the length of the lesion; and finally, for lesions at the flexures, using a clear cap similar to cap-assisted EMR to obtain a more en face view as well as a sphincterotome in order to orient the catheter in the direction of the lumen [8]. Right colonic stenting always presents a challenge given the risk of perforation. Helpful tips include but are not limited to the following: using hydrophilic soft-tipped or narrow caliber (0.025-in.) biliary guidewires and inserting them into a standard biliary catheter to cannulate the stricture; using a colonoscope with a 4.2-mm working channel in order to limit friction during passage of the predeployed stent if excessive sigmoid looping occurs; and finally, using longer stents (90 mm) in areas of tortuosity [11].
Pitfalls
There are many challenges and pitfalls of endoscopic colonic stenting. Tumors can be located at colonic angulations or flexures resulting in lack of en face visualization [8]. Patients with an indication for colonic stenting such as those with acute colonic obstruction are acutely ill and often cannot be adequately prepped [8]. Also, there is a relative unfamiliarity of the fluoroscopic nuances of the colon compared to other areas of the GI tract [8]. Balloon dilation of the stricture before or after SEMS placement is discouraged given increased risk of perforation [8]. Complete or near complete obstruction presents many pitfalls, and one should consider using carbon dioxide, water, or the least amount of air for insufflation given the risk of perforation [8]. Additionally, consideration should be given to leaving the guidewire in place after stent deployment to pass a colonic decompression tube through the stent and into the proximal bowel if immediate passage of stool does not occur after stent placement [8]. The thin-walled right colon intrinsically has a higher risk of perforation, and in the past, acute obstruction of the right colon was handled by resection and primary anastomosis [11]. More recent reports suggest that an emergent right colonic resection has high morbidity and mortality and stent placement can be beneficial in transforming the procedure into an elective one with lower surgical risk [11].
Does Location of Obstruction Affect Stent Success?
Left-sided obstructions are located away from the left flexure, while proximal obstructions are located between the ileocecal valve and the left flexure. In Tal et al. [12], the outcomes of through-the-scope (TTS) implanted SEMS in both locations were compared in terms of complications and outcome in palliation of the colonic obstruction. In this 15-patient study [12], the location of the SEMS placement did not impact the outcome or the complication rate. SEMS patency duration was 269.8 ± 175.2 days, and the mean survival of patients was 305.1 ± 279 days [12]. In a review of 21 patients with right-sided malignant obstructions [11], the technical success rate was 95 %, with 85 % of patients achieving complete relief of obstruction. There were no procedure-related complications, and the authors felt that the success rates were similar to those of distal colonic stenting [11].
Types of Stents
There are a number of dedicated colonic stents available for palliative and preoperative decompression purposes. The Enteral Wallstent™ (Boston Scientific, Natick, Massachusetts, USA) is delivered using a 10-French through-the-scope (TTS) delivery system that fits through a 3.7-mm working channel of an endoscope [6]. The deployed stent diameter is 20 or 22 mm and the length is 60 or 90 mm [6]. Newer devices include the Wallflex™ colonic stent that comes in a larger diameter (25 mm) and longer length (60, 90, or 120 mm) that can be delivered TTS or over a guidewire and the Ultraflex™ Precision Colonic Stent System (Boston Scientific, Natick, Massachusetts, USA) that comes with a larger diameter (25 mm) and longer length (57, 87, or 117 mm) that is delivered over a wire [6]. The Wallflex™ is made of Elgiloy that is capable of generating high radial forces [7]. The larger diameter prevents occlusion with fecal matter [6]. The Ultraflex™ is made of nitinol, which has increased flexibility that aids with stenting regions of sharp angulation at the cost of lesser radial force [7]. Colonic Z-stent® (Wilson-Cook, Winston-Salem, North Carolina, USA) is made from stainless steel with a 35-mm flare surrounding a 25-mm diameter stent and comes in a variety of lengths (40, 60, 80, 100, and 120 mm) [6]. The flare, whether proximal or distal, helps to prevent migration [7]. Stents are produced in coated and uncoated versions [7]. Coated versions have lower rates of tissue ingrowth but have a higher rate of migration [7]. Only uncoated stents are approved for use in the colon [7].
In Small et al. [13], the Enteral Wallstent was compared with the newer Ultraflex stent in the palliation of malignant left-sided colonic obstruction. Delayed complications (perforation, stent occlusion, migration, and erosion) were seen more with the Enteral Wallstent than the Ultraflex (38 % vs. 20 %). Thus, reintervention was needed more frequently for Enteral Wallstent than for Ultraflex (40 % vs. 17 % endoscopically and 46 % vs. 26 % operatively). In Van Hooft et al. [14], the new Wallflex colonic stent was compared to surgical intervention in stage IV left-sided colorectal cancer. The study was prematurely closed due to high number of serious adverse outcomes, primarily perforations, in the stenting group. The perforations may have been related to the Wallflex itself or its use in the setting of chemotherapy.
Multiple studies have compared covered and uncovered SEMS especially in the setting of malignant colonic obstructions. In Choi et al.’s retrospective study with 152 patients [15], covered stents were found to be an independent risk factor for complications along with complete obstruction. The technical success rate was 100 %, and the clinical success rate was 94.1 % [15]. In Lee et al.’s prospective study with 80 patients [16], there was no difference in early stent migration rate between the two groups, but late stent migration was more common in the covered stent group (0 % vs. 40 %, P = 0.005). Loss of stent function in long-term follow-up was more common in the covered stent group (18.8 % vs. 60 %, P = 0.018) [16]. In Sebastian et al. [17], the reobstruction rate in the covered stent group was significantly lower than in the uncovered stent group (4.7 % vs. 7.81 %, P = 0.003).
Most recently, the biodegradable polydioxanone stent, which was initially developed for the treatment of refractory benign esophageal strictures, was used in the colon [18]. It was used successfully in a Crohn’s disease patient with a sigmoid colon stricture not amenable to balloon dilation [18]. These biodegradable stents have the advantage of not needing to be removed, but further studies are needed to verify their safety and efficacy in the colon [18].
Indications
Colonic stenting has been indicated primarily for palliation of advanced disease and preoperative decompression. Preoperative decompression allows for a normally emergent two-step operation which often includes the placement of a colostomy to be converted to an elective one-step surgery. The avoidance of an emergency operation allows for optimization of the patient for an elective operation, often performed laparoscopically. Certainly, emergency surgery has a role in the event of ischemia and/or perforation. Less common indications for colonic stenting are benign strictures and extracolonic malignancy leading to colonic obstruction.
Use in Colonic Malignancy
As a Bridge to Surgery
Stenting obstructions in colorectal carcinoma can serve as a bridge to an elective operation and lead to lower complication rates compared to those of emergent surgery. In Sebastian et al. [17], stenting as a bridge to surgery achieved technical success in 92 % of cases and clinical success in 85 % of cases. Of those that had clinical success, 95 % went on to one-stage colon resection within a mean of 8.9 days (range 2–115 days) [17]. Stent-related complications included perforation (occurring in 4 % and associated with balloon predilection), stent migration (10 %), and reobstruction (10 %) [17].
In Ghazal et al. [19], 96.7 % underwent successful stenting and had elective surgery 7–10 days later. Complications were seen in 13.8 % of the stenting group compared to 50 % in the emergency surgery group with more frequent bowel movements per day in the first three postoperative months [19]. In Watt’s review of 88 articles [7, 20], the technical and clinical success rates of stenting were in excess of 90 %, and the median time to elective surgery after stenting was 5.8 days (range 2–16 days). Stenting followed by elective surgery appeared safer than emergency surgery with higher rates of primary anastomosis, lower rates of colostomy, shorter hospital stay, and lower overall complication rates [7, 20].
In 5 randomized trials identifying 207 participants [19], the average time to clinical relief of obstruction was 0.66 days in the colonic stent group and 3.55 days in the emergency surgery group, with no statistically significant difference in 30-day mortality or in overall complication rate. The stent-related perforation rate was 5.88 %, the migration rate was 2.13 %, and the obstruction rate was 2.13 %. The advantages of colorectal stenting were shorter hospital stay and procedure time and less blood loss.
As Palliation
Colonic obstruction can occur in stage IV disease treated nonoperatively with chemotherapy with or without radiation, as well as at the site of prior resection [2]. In Meisner et al. [21], the Wallflex uncovered enteral colonic stents were used to relieve obstruction in patients with incurable disease and as a way to avoid palliative stoma surgery. Clinical success rates were 87.8 % at 30 days and 96 % at 12 months [21]. Perforation rate was 5.1 % and migration rate was 5.5 % [21]. Overall death rate was 48.6 % of which close to 70 % were cancer-related deaths and only 2 deaths were attributed to stent-related complications out of the 255 patients stented [21].
In Shrivastava et al. [22], the Wallflex or Memotherm® stent was inserted for palliation over an 8-year period from 1998 to 2006. Technical success was achieved in 89 % and clinical success in 99 % of patients. At the time of analysis in 2006, 14.2 % of the patients were alive, and of those, the median survival was 59 days. 8.6 % had stent migration, which occurred 10.3 days after insertion, and 3.7 % suffered perforation.
In Tilney et al.’s review of ten studies [23], stent insertion was attempted in 54.1 % of 451 patients and was successful in 92.6 %. In the stent group, compared to the surgically treated group, the patients had shorter length of hospital stay by 7.72 days, lower mortality, and fewer complications [23]. Stoma formation was significantly lower in the stent group [23]. Colonic stenting offered effective palliation for malignant bowel obstruction with short lengths of hospital stay and low rate of stoma formation [23].
Use in Benign Strictures
Patients with benign colonic strictures may benefit from stent placement as it offers immediate nonoperative colonic decompression, but stents may need to last considerably longer than when used for malignancy. Benign strictures are not an established indication for stent placement, and little data exists on stent decompression of benign strictures. Benign strictures may be due to diverticular, inflammatory, anastomotic, postischemic, or postradiation changes [2]. Benign acute colonic obstructions that are at high risk of emergency surgery can gain temporary relief of obstruction after SEMS placement [2]. The stent can then be removed en bloc with the resected colon [2]. Higher rates of stent migration are seen with benign strictures. Inflammatory strictures such as with diverticular disease may cause stent migration from resolution of the inflammation of the bowel wall [24]. Radiation-induced strictures may cause migration from the stent not being able to embed in the mucosa that is damaged [24].
Suzuki et al. [25] had a high stent migration rate of 19.4 % in benign disease, though stenting was still effective in providing luminal patency at a median follow-up of 7.5 months. In Vanbiervliet et al. [26], fully covered stents were placed in 43 patients with symptomatic benign colonic strictures. Migration was observed in 63 %, and stents more than 20 mm wide migrated significantly less often [26]. The median duration of stenting was 21 days [26]. Recurrence of obstruction occurred in 53 % of patients within the 4–6-week follow-up period [26].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








