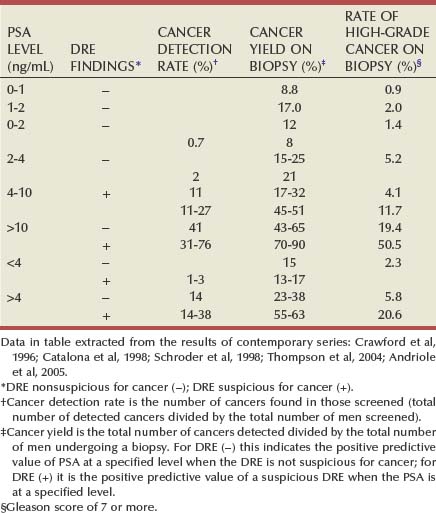
Stacy Loeb, MD, Herbert Ballentine Carter, MD This chapter will review diagnostic and staging modalities for prostate cancer. Screening refers to testing for disease in healthy, asymptomatic populations, whereas diagnosis is the identification of disease among individuals with signs or symptoms. The principal goal of screening is to improve overall health outcomes by identifying and treating disease at an earlier stage. Despite the common misperception that early diagnosis through screening is invariably beneficial, it also has the potential for harm (Welch, 2004). There is strong evidence that prostate cancer screening reduces the rates of advanced disease (van der Cruijsen-Koeter et al, 2006; Aus et al, 2007), but controversy exists regarding the balance of benefit and harm (Barry, 2009). From 1993 to 2003 after the onset of widespread PSA testing, the mortality rate from prostate cancer declined by 32.5% (Surveillance, Epidemiology, and End Results [SEER] Program), along with a 75% reduction in the proportion of advanced-stage disease at diagnosis. It was estimated that PSA screening could have accounted for 45% to 70% of this reduction in prostate cancer mortality in the United States (Etzioni et al, 2008). However, randomized trials comparing the disease-specific outcomes of men who are screened and those who are not represent the highest level of evidence for screening. The Prostate, Lung, Colon, and Ovary (PLCO) trial of the National Cancer Institute (NCI) and the European Randomized Trial of Prostate Cancer Screening (ERSPC) were initiated in 1993 to compare prostate cancer–specific mortality (primary end point) between screened and unscreened arms (Auvinen et al, 1996; de Koning et al, 2002; Schroder, 2003; Andriole et al, 2004). Reductions in high-grade cancer and locally advanced/metastatic disease with serial screening were reported in the ERSPC (van der Cruijsen-Koeter et al, 2006; Aus et al, 2007), as well as a 20% reduction in prostate cancer–specific mortality among those screened compared with controls at a median follow-up of 9 years (Schroder et al, 2009). However, in the ERSPC, it was estimated that to prevent one prostate cancer death would require screening 1410 men and treating an additional 48 men. With longer follow-up, the Goteborg randomized population-based screening trial reported a greater mortality benefit with screening. By comparison, there was no difference in prostate cancer mortality between the screened and control arms of the PLCO at a median follow-up of 11 years (Andriole et al, 2009). Both trials reported results early when considering the long natural history of prostate cancer; and, with further follow-up, the findings could change. Further, the disparate findings between the studies may result from high rates of screening in the control arm of the PLCO (contamination) and lesser ability to detect a mortality difference given that the PLCO had approximately threefold fewer events than the ERSPC (Barry, 2009). These randomized trials emphasize the potential for overdiagnosis (detection of cancers that would have otherwise remained undetected) and overtreatment of prostate cancer with screening. Overtreatment is especially concerning among older men (age greater than 65 years), for whom treatment was associated with minimal benefit in a randomized trial of surgery versus watchful waiting (Bill-Axelson et al, 2008). Given that the average age at diagnosis today is approximately 67 years, the risk of overtreatment is high. Professional groups have published statements and guidelines on prostate cancer screening (National Comprehensive Cancer Network, 2007; Lim and Sherin, 2008; Lin et al, 2008; U.S. Preventive Services Task Force, 2008). The U.S. Preventive Services Task Force (2008) concluded that the evidence is insufficient to assess the balance of benefit and harm of screening among men age 75 years or less, whereas screening is not recommended for men age 75 years or older. The American College of Preventive Medicine recommends against routine population screening with PSA and DRE (Lim and Sherin, 2008) and suggests shared decision making for men age 50 years or older with a life expectancy greater than 10 years. The American Cancer Society and the American Urological Association recommend annual prostate cancer screening beginning at age 50 years for average-risk men and earlier for higher-risk men (positive family history or black race). The National Comprehensive Cancer Network (2007) recommends offering baseline PSA screening at age 40 years with the frequency of follow-up testing based on PSA test results. The appropriate age to start and discontinue screening (Ross et al, 2005; Catalona et al, 2006; Schaeffer, 2009) and the appropriate interval between screens will continue to be a matter of debate (Carter et al, 1997; Ross et al, 2000; Hugosson et al, 2003b). Despite the controversy associated with prostate cancer screening and disparate recommendations from professional organizations, opportunistic prostate cancer screening is highly prevalent in the United States (Lu-Yao et al, 2003; Sirovich et al, 2003; Ross et al, 2004; Schwartz et al, 2004; Chan et al, 2006; Walter et al, 2006). Although prostate cancer screening remains controversial, men who present for periodic health examinations should be made aware of the availability of the PSA test so that they can make an informed decision whether or not to be screened. Before the availability of PSA, physicians relied solely on DRE for early detection of prostate cancer (Cooner et al, 1990; Catalona et al, 1994; Ellis et al, 1994; Schroder et al, 1998; Vis et al, 2001; Okotie et al, 2007). DRE has only fair reproducibility in the hands of experienced examiners (Smith and Catalona, 1995) and misses a substantial proportion of early cancers (Cooner et al, 1990; Catalona et al, 1994; Ellis et al, 1994). It has been suggested that the value of DRE for screening at PSA levels less than 3.0 ng/mL is limited (Schroder et al, 1998; Vis et al, 2001). Due to the risk of prostate cancer among men with abnormalities on DRE and the simplicity of the examination, most urologists use PSA and DRE together for prostate cancer detection. Further, PSA improves the positive predictive value of DRE for cancer (Schroder et al, 1998). The positive predictive value of DRE ranged from 4% to 11% in men with PSA levels of 0 to 2.9 ng/mL, and from 33% to 83% in men with PSA levels of 3 to 9.9 ng/mL or more (Schroder et al, 1998). Overall, when DRE and PSA are used in prostate cancer screening, detection rates are higher with PSA than with DRE and highest with both tests together (Catalona et al, 1991). Because DRE and PSA do not always detect the same cancers (Okotie et al, 2007), the tests are complementary and are recommended in combination as methods of assessing prostate cancer risk. Serum PSA levels vary with age, race, and prostate volume. Blacks without prostate cancer have higher PSA values than whites (Morgan et al, 1996; Fowler et al, 1999). PSA increases 4% per milliliter of prostate volume; and 30% and 5% of the variance in PSA can be accounted for by prostate volume and age, respectively (Oesterling et al, 1993). PSA expression is strongly influenced by androgens (Young et al, 1991; Henttu et al, 1992). Serum PSA becomes detectable at puberty with increases in luteinizing hormone and testosterone (Vieira et al, 1994). In hypogonadal men with low testosterone levels, serum PSA may be low because of decreased expression and may not reflect the presence of prostate disease such as cancer (Morgentaler et al, 1996). Metabolic factors can influence the serum PSA concentration. Obese men have slightly lower PSA levels than nonobese men (Baillargeon et al, 2005), possibly due to hemodilution (Banez et al, 2007). Statin use may reduce PSA levels by lowering lipids (Hamilton et al, 2008). Overall, the presence of prostate disease (prostate cancer, benign prostatic hyperplasia [BPH], and prostatitis) is the most important factor affecting serum PSA levels (Wang et al, 1981; Ercole et al, 1987; Dalton, 1989; Nadler et al, 1995). Although PSA elevations may indicate the presence of prostate disease, not all men with prostate disease have elevated PSA levels, and PSA elevations are not specific for cancer. It is postulated that serum PSA elevations occur from disruption of the normal prostatic architecture, allowing PSA to gain access to the circulation. This can occur in the setting of prostate disease (BPH, prostatitis, prostate cancer) and with prostate manipulation (e.g., prostate massage, prostate biopsy, transurethral resection) (Klein and Lowe, 1997). Although DRE can lead to slight increases in serum PSA, the resultant change in PSA falls within the error of the assay and rarely causes false-positive tests (Crawford et al, 1992). Studies examining the effect of ejaculation on serum PSA have reported conflicting results (Simak et al, 1993; Kirkali et al, 1995; Tchetgen et al, 1996; Heidenreich et al, 1997; Herschman et al, 1997; Stenner et al, 1998; Yavascaoglu et al, 1998). A repeat PSA after 48 hours of sexual abstinence may be helpful for interpreting serum PSA levels that are minimally elevated. Prostate-directed treatments (for BPH or prostate cancer) can lower serum PSA by decreasing the volume of prostatic epithelium available for PSA production and by decreasing the amount of PSA produced per cell (Shingleton et al, 2000). 5α-Reductase inhibitors that are used for BPH treatment have been shown to lower PSA levels, including both type 2 isoenzyme inhibitors (finasteride) and dual type 1 and 2 isoenzyme inhibitors (dutasteride) (Guess et al, 1993; Roehrborn et al, 2002). Finasteride 1 mg (trade name Propecia) used for male pattern hair loss (androgenic alopecia) results in the same decline in serum PSA levels as the 5-mg dosage (D’Amico and Roehrborn, 2007). Men initiating treatment with 5α-reductase inhibitors should first have a baseline PSA measurement and should be followed with serial measurements. Although the PSA level is often multiplied by two to estimate the “true” PSA level of a patient who has been taking a 5α-reductase inhibitor for 12 months or more (Andriole et al, 1998), the PSA response to finasteride treatment can be highly variable (Marks et al, 2006). It has been suggested that the PSA should instead be multiplied by a factor of 2.3 after 2 years and 2.5 after 7 years of treatment (Etzioni et al, 2005; Thompson et al, 2007; Walsh, 2008). Because this “moving target” can complicate the use of PSA in daily clinical practice, some have recommended using the PSA nadir on finasteride as the new baseline and performing a biopsy for subsequent PSA increases (Morgentaler, 2007). Surgical therapy for BPH can lead to reductions in the serum PSA level (Shingleton et al, 2000) and “reset” the PSA baseline to a variable extent by removing the main contributor to PSA (transition zone). The initial assays for PSA that were approved by the FDA in 1994 for early detection of prostate cancer detected both free PSA and PSA complexed to alpha 1 antichymotrypsin (ACT). Thus measurement of free and complexed PSA by these assays is generally referred to as the serum PSA level (Smith et al, 1996). Specific assays that detect free PSA alone and PSA complexed to ACT alone have been evaluated and approved for prostate cancer detection (see later). It is now well-established that the use of PSA increases the detection of prostate cancers that are more likely to be organ-confined when compared with detection without PSA (Thompson et al, 1987; Mueller et al, 1988; Chodak et al, 1989; Rietbergen et al, 1999; Hoedemaeker et al, 2000). Observational studies and randomized trials have shown that both the future risk of prostate cancer and the chance of finding cancer on a prostate biopsy increase incrementally with the serum PSA level (Catalona et al, 1991, 1994; Brawer et al, 1992; Labrie et al, 1992; Gann et al, 1995; Fang et al, 2001; Thompson et al, 2004; Andriole et al, 2005; Whittemore et al, 2005; Loeb et al, 2006; Lilja et al, 2007). Gann and associates (1995) were the first to demonstrate the association between the baseline PSA level and subsequent prostate cancer detection, which has been verified by others (Fang et al, 2001; Antenor et al, 2004; Whittemore et al, 2005; Loeb et al, 2006). In addition to predicting future risk, PSA is directly associated with the present risk of prostate cancer. The probability of detecting prostate cancer on biopsy increases directly with PSA across the full spectrum of PSA levels (Table 99–1) (Thompson et al, 2004). Table 99–1 Prostate Cancer Detection as a Function of Serum Prostate-Specific Antigen (PSA) Level and Digital Rectal Examination (DRE) Findings in Contemporary Series The choice of a PSA threshold for recommending a prostate biopsy is controversial (Catalona et al, 1994; Gann et al, 1995; Carter, 2004; Nadler et al, 2005; Thompson et al, 2005) and has recently been reviewed (Schroder et al, 2008). Gann (1995) pointed out that “dichotomization of PSA results into normal and abnormal obscures important information contained in levels below the usual cutoff.” Data from the Prostate Cancer Prevention Trial clearly show that the risk of prostate cancer is continuous as PSA increases (Thompson et al, 2005). Some investigators have recommended against referring to PSA as “elevated” or “abnormal,” and instead they advise using PSA together with other methods of risk assessment, such as family history, race, and DRE findings (Thompson et al, 2006). Nevertheless, most urologists continue to use total PSA thresholds for prostate biopsy decisions. A PSA level that is considered suspicious for prostate cancer should be remeasured before performing a prostate biopsy, because of fluctuations in PSA that could create false-positive elevations (Eastham et al, 2003). Numerous variations on PSA-based screening have been proposed to improve test performance, including the adjustment of the PSA level for total prostate volume (PSA density) (Babaian et al, 1990; Veneziano et al, 1990; Littrup et al, 1991; Benson et al, 1992a, 1992b; Bazinet et al, 1994; Rommel et al, 1994; Catalona et al, 2000; Djavan et al, 2002; Egawa et al, 2002; Naya et al, 2002; Gjengsto et al, 2005) or transition zone volume (Djavan et al, 1999a, 1999b; Taneja et al, 2001; Singh et al, 2004; Gjengsto et al, 2005), and the evaluation of rate of change in PSA (PSA velocity) (Carter et al, 1992; Smith and Catalona, 1994; Fowler et al, 2000; Fang et al, 2002; D’Amico et al, 2004; Roobol et al, 2004; Berger et al, 2005; D’Amico et al, 2005; Schroder et al, 2006; Berger et al, 2007; Loeb et al, 2007a, 2007b, 2008c; Eggener et al, 2008; Vickers et al, 2009). The discovery that PSA circulates in both bound (complexed) and unbound (free) forms and development of assays to measure these forms separately, have resulted in the investigation of their use for prostate cancer detection (McCormack et al, 1995; Lilja, 1997, 2003; Polascik et al, 1999; Gretzer and Partin, 2003) and this topic has recently been reviewed (Jansen et al, 2009). Distinguishing between men with PSA elevations driven by BPH or cancer is difficult, because PSA is not specific for cancer and the prevalence of BPH is high. Volume-based PSA parameters (with prostate volume typically determined by ultrasonography) have been evaluated to reduce confounding from BPH. These include PSA density (PSAD, PSA divided by prostate volume), complexed PSA density (complexed PSA divided by prostate volume), and PSA transition zone density (PSA divided by transition zone volume) (Babaian et al, 1990; Veneziano et al, 1990; Littrup et al, 1991; Benson et al, 1992a, 1992b; Bazinet et al, 1994; Rommel et al, 1994; Djavan et al, 1999a, 1999b, 2002; Catalona et al, 2000; Naya et al, 2002; Gjengsto et al, 2005). Benson and colleagues (1992a, 1992b) suggested that adjusting PSA for prostate size by dividing PSA by prostate volume (PSAD) could help distinguish between PSA elevations caused by BPH and those caused by prostate cancer. A direct relationship between PSAD and the chance of cancer has been documented (Seaman et al, 1993; Bazinet et al, 1994; Rommel et al, 1994), and a PSAD of 0.15 or greater was proposed for recommending prostate biopsy in men with PSA levels between 4 and 10 ng/mL and a normal DRE (Seaman et al, 1993; Bazinet et al, 1994). The usefulness of PSAD in prostate cancer detection has not been confirmed in all studies (Cooner, 1994; Taneja et al, 2001). An advantage of PSAD is that it has been directly associated with prostate cancer aggressiveness (Carter et al, 2002, 2007; Kundu et al, 2007). PSA has been adjusted for the transition zone volume (Kalish et al, 1994), the prostatic region that is the major determinant of serum PSA in men without prostate cancer (Lepor et al, 1994). Djavan and associates (1999b) found that PSA transition zone volume was the parameter with the highest overall sensitivity and specificity for prostate cancer detection when PSA was between 4 to 10 ng/mL. In general, although PSAD and its associated measures are imperfect predictors of cancer, they represent an additional method of risk assessment with potential utility for counseling men with intermediate PSA levels (4 to 10 ng/mL) regarding the need for prostate biopsy (Benson and Olsson, 1994) or repeat biopsy if PSA is persistently elevated (Keetch et al, 1996). Short-term fluctuations in PSA can occur between measurements in the presence or absence of prostate cancer, primarily due to physiologic variation (Carter et al, 1992, 1995; Riehmann et al, 1993; Prestigiacomo and Stamey, 1996; Eastham et al, 2003). However, the rate of change in PSA (PSA velocity, or PSAV)—PSA corrected for the elapsed time between measurements (Carter et al, 1992)—is associated with the risk of prostate cancer (Carter et al, 1992; Smith and Catalona, 1994; D’Amico et al, 2004, 2005; Roobol et al, 2004; Berger et al, 2005, 2007; Sengupta et al, 2005; Schroder et al, 2006; Loeb et al, 2007a, 2007b, 2008a; Eggener et al, 2008; Vickers et al, 2009). Using frozen sera to measure PSA years before diagnosis, Carter and colleagues (1992) showed that a PSAV more than 0.75 ng/mL per year was a specific marker for the presence of prostate cancer in men with PSA levels between 4 and 10 ng/mL. Other studies have demonstrated that men with prostate cancer have more rapid rises in PSA than men without prostate cancer (Smith and Catalona, 1994; Carter et al, 1995; Raaijmakers et al, 2004; Thompson et al, 2004; Loeb et al, 2008a). More recently, it has been shown that PSAV might be useful for prostate cancer detection among men with PSA levels less than 4.0 ng/mL (Carter et al, 2006; Loeb et al, 2007b). Some investigators have suggested the use of lower PSAV thresholds for men with lower total PSA levels (Loeb et al, 2007b; Moul et al, 2007). Some studies have failed to demonstrate the value of PSAV for prostate cancer prediction beyond that of a single PSA measurement (Roobol et al, 2004; Vickers et al, 2009). Differences between studies could be due to the method of calculating PSAV (Yu et al, 2006; Connolly et al, 2007) and the point in the PSA history at which PSAV is calculated (Carter et al, 1992; D’Amico et al, 2004). PSAV may play a role in the prediction of life-threatening prostate cancer (D’Amico et al, 2004, 2005; Carter et al, 2006; Loeb et al, 2008a, 2008b). A PSAV greater than 0.35 ng/mL/year 10 to 15 years prior to diagnosis was associated with a fivefold increased risk of life-threatening prostate cancer more than a decade later (Carter et al, 2006), while a PSAV greater than 2 ng/mL/year during the year prior to a prostate cancer diagnosis was associated with prostate cancer–specific mortality following radical prostatectomy or radiation therapy (D’Amico et al, 2004, 2005; Sengupta et al, 2005). However, a recent meta-analysis suggested that PSAV prior to treatment provides no additional information regarding prostate cancer outcome when compared with PSA alone (Vickers et al, 2009). Men with prostate cancer generally have a greater fraction of serum PSA that is complexed—and therefore a lower percentage of total PSA circulating in the free (unbound) form—than men without prostate cancer (Christensson et al, 1993; Leinonen et al, 1993; Lilja, 1993; Stenman et al, 1994; Catalona et al, 1995, 1998, 2000; Keetch et al, 1997; Pannek et al, 1998; Woodrum et al, 1998; Gann et al, 2002; Roehl et al, 2002; Hugosson et al, 2003a; Raaijmakers et al, 2004). This difference is thought to be due to differential expression of PSA isoforms by transition zone (zone of origin of BPH) tissue compared with peripheral zone tissue (where most prostate cancers arise) (Chen et al, 1997; Mikolajczyk et al, 1997, 2000a, 2000b). Free PSA levels vary directly by age and prostate volume, and vary indirectly with the total PSA level (Woodrum et al, 1998). In addition, because assays differ in their ability to determine both free and total PSA, results may differ depending on the assay or combination of assays used (Woodrum et al, 1998). The percentage of free PSA (%fPSA) does not appear to be significantly altered by race (Catalona et al, 2000) or 5α-reductase inhibitors (Keetch et al, 1997; Pannek et al, 1998). %fPSA has been shown to significantly improve the ability to distinguish between individuals with and without prostate cancer, compared with total PSA alone (Christensson et al, 1993). The %fPSA cutoff that optimizes sensitivity and specificity for cancer detection depends on prostate size, because overlap is greatest among men with enlarged prostates, with or without concomitant prostate cancer (Catalona et al, 1995). %fPSA appears to be most useful in distinguishing between those with and without prostate cancer at intermediate total PSA levels. In men with PSA levels of 4 to 10 ng/mL and palpably benign prostate glands, a %fPSA cutoff of 25% detected 95% of cancers while avoiding 20% of unnecessary biopsies (Catalona et al, 1998). The value of %fPSA to predict prostate cancer at levels less than 4.0 ng/mL is unclear (Gann et al, 2002; Roehl et al, 2002; Hugosson et al, 2003a; Raaijmakers et al, 2004). %fPSA (at a cutoff of 25%) and PSAD (using a threshold of 0.078) have been shown to have comparable specificity (at a sensitivity of 95%) although %fPSA does not require transrectal ultrasonography (TRUS) (Catalona et al, 2000). Thus %fPSA can be used to counsel men with PSA elevations in the range 4 to 10 ng/mL regarding their risk of cancer. Because men with prostate cancer have a greater fraction of total PSA that is complexed to protease inhibitors than men without prostate cancer, measurement of complexed PSA (cPSA) has been studied as a marker for detection (Brawer et al, 2000, 2002; Okegawa et al, 2000; Parsons et al, 2004). When total PSA levels were between 4 and 10 ng/mL, cPSA provided improved specificity compared with total PSA, and similar specificity compared with the percentage of free PSA at a sensitivity of 95% (Brawer et al, 2000), findings that were subsequently confirmed (Okegawa et al, 2000). Similar results were reported in the 2.6 to 4.0 ng/mL PSA range (Parsons et al, 2004). Overall, at a high sensitivity, cPSA provides higher specificity compared with total PSA and comparable specificity to %fPSA in prostate cancer detection. A potential advantage of cPSA is the requirement for one assay. PSA is secreted from the prostatic luminal epithelium in a precursor form (pPSA or proPSA) (see Chapter 98) (Mikolajczyk et al, 2001, 2004; Peter et al, 2001; Catalona et al, 2003, 2004; Gretzer and Partin, 2003; Khan et al, 2003; Lilja, 2003; Canto et al, 2004; Lein et al, 2005; Makarov et al, 2009). Active free PSA can be further cleaved to BPSA or intact PSA (iPSA) that is inactive and not complexed. Research assays have been developed for measuring BPSA and pPSA (both native and truncated forms). The relative concentration of these isoforms differs in the presence of prostatic disease. BPSA is found preferentially in nodular BPH tissue from the transition zone and can be considered a marker for BPH (Mikolajczyk et al, 2000b; Canto et al, 2004), whereas a larger relative proportion of proPSA has been associated with prostate cancer (Mikolajczyk et al, 1997, 2000a, 2001; Peter et al, 2001). Some studies have suggested that proPSA may improve the identification of prostate cancer among men with PSA levels of 2 to 4 ng/mL (Catalona et al, 2003, 2004), 4 to 10 ng/mL (Khan et al, 2003; Mikolajczyk et al, 2004), and 2 to 10 ng/mL (Catalona et al, 2003), while other studies have not shown incremental predictive value of specific subtypes beyond %fPSA (Lein et al, 2005). hK2 is a closely related serine protease in the PSA/kallikrein gene family that has also been evaluated for prostate cancer detection (Kwiatkowski et al, 1998; Partin et al, 1999; Becker et al, 2000, 2003; Haese et al, 2003; Bangma et al, 2004; Steuber et al, 2005; Vickers et al, 2008). Expression of hK2 is higher in more poorly differentiated cancer tissues than in normal and benign tissues (Tremblay et al, 1997). Although some studies have suggested that the ratio of hK2 and free PSA might improve the ability of PSA to identify men with prostate cancer (Kwiatkowski et al, 1998; Partin et al, 1999; Becker et al, 2000; Vickers et al, 2008), other analyses have not (Becker et al, 2003; Bangma et al, 2004). hK2 does appear to correlate directly with grade and cancer volume and could be useful in patient assessment after diagnosis (Haese et al, 2003; Steuber et al, 2005). Prostate cancer gene 3 (PCA-3) is a noncoding prostate-specific mRNA overexpressed in prostate cancer tissue compared with benign tissue (Bussemakers et al, 1999; Marks et al, 2007; Deras et al, 2008; Haese et al, 2008; Nakanishi et al, 2008; Sokoll et al, 2008; van Gils et al, 2008; Whitman et al, 2008). Urine assays have been developed to measure PCA-3 mRNA (Sokoll et al, 2008), which is associated with the likelihood of a positive initial or repeat prostate biopsy (Marks et al, 2007; Deras et al, 2008; Haese et al, 2008). There are conflicting results on the association between PCA-3 with prostate cancer aggressiveness (Nakanishi et al, 2008; van Gils et al, 2008; Whitman et al, 2008). In the future, it is likely that panels of biomarkers will be used in combination with standard measures of risk (age, family history, race) to selectively identify men who should undergo further evaluation for the presence of prostate cancer (Etzioni et al, 2003). Clinical staging is the assessment of disease extent using pretreatment parameters (DRE, PSA, needle biopsy findings, and radiologic imaging); whereas pathologic stage is determined after prostate removal and involves histologic analysis of the prostate, seminal vesicles, and pelvic lymph nodes if lymphadenectomy is performed. Pathologic staging more accurately estimates disease burden and is more useful than clinical staging for outcome prediction (Pound et al, 1997). Biochemical recurrence-free survival and cancer-specific survival are both inversely related to the pathologic stage of disease (Roehl et al, 2004). The most important pathologic criteria that predict prognosis after radical prostatectomy are tumor grade, surgical margin status, extracapsular disease, seminal vesicle invasion, and pelvic lymph node involvement (Jewett, 1975; Walsh and Jewett, 1980; Epstein et al, 1990, 1993a, 1993b; Partin et al, 1993; Pound et al, 1997). The Whitmore and Jewett staging system is now of historical interest (Jewett 1956; Whitmore 1956). Today, clinical staging is based upon the tumor-node-metastases (TNM) classification system (Table 99–2). This system was first adopted in 1975 by the American Joint Committee on Cancer (AJCC) and has since undergone numerous modifications (Schroder et al, 1992). The most recent 1997 modification reduced the subdivision of T2 disease from three categories (T2a, T2b, and T2c) to two categories by combining single-lobe disease (T2a and T2b) into a single stage (Schroder et al, 1992). However, some believe that a distinction between stages T2a and T2b is clinically important (Iyer et al, 1999; Han et al, 2000). Also, a nonpalpable lesion identified by TRUS is considered T2 by the current TNM clinical staging system. However, TRUS findings do not predict tumor extent in PSA-detected nonpalpable lesions (Epstein et al, 1994; Ferguson et al, 1995) so that many urologists classify men with nonpalpable disease as T1c regardless of TRUS findings. Table 99–2 1997 and 1992 TNM Clinical Staging Systems for Prostate Cancer
Early Detection
Screening
General Concepts of Screening
Randomized Trials
Specialty Group Recommendations
Diagnostic Modalities
Digital Rectal Examination
Prostate-Specific Antigen (PSA)
Factors Influencing PSA
Clinical Use for Diagnosis
Triggers for Biopsy
PSA Derivatives and Molecular Forms
Volume-Based PSA Parameters
Prostate-Specific Antigen Velocity
Free Prostate-Specific Antigen
Complexed Prostate-Specific Antigen
PSA Isoforms
hK2
Other Markers (see Chapter 98)
Staging
General Concepts of Staging
Clinical versus Pathologic Staging
Classifications
1997
1992
DESCRIPTION
TX
TX
Primary tumor cannot be assessed
T0
T0
No evidence of primary tumor
T1
T1
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access




