Chapter 100 Early and late complications of liver transplantation
Overview
Liver transplantation has evolved from a risky procedure with high morbidity and mortality to a standard treatment for patients with liver failure. Patients who undergo successful liver replacement have 5-year survival rates that exceed 70% (Muraji et al, 1997; see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ). Despite this dramatic improvement in outcome, a significant percentage of patients experience life-threatening complications that can result in the need for reoperation. As expertise in the procedure grows, surgeons are willing to attempt liver transplantation in patients who previously were considered poor candidates for surgery. Recipient portal vein thrombosis (PVT) was considered an absolute contraindication to surgery, but now transplantation is commonly performed in the presence of PVT (Shaked & Busuttil, 1991; Stieber et al, 1991).
As surgeons continue to develop new surgical procedures, such as transplantation of reduced-size and split-liver grafts and living-donor liver transplantation (LDLT), a new series of complications unique to these procedures is emerging (Broelsch et al, 2000; Emond et al, 1993). The incidence of hepatic artery thrombosis, bile leaks, and stricture is at least two times higher in patients who receive living-donor grafts compared with those who receive cadaveric grafts (Malago et al, 2003). Despite a higher morbidity rate in recipients of living-donor grafts, patient and graft survival are similar or superior to those observed with deceased donors (Fan et al, 2002; Lo et al, 2002; Pomposelli et al, 2006). This chapter reviews common early and late complications encountered during and after liver transplantation. Because complications after liver transplantation represent a continuum, most can occur at any time after surgery.
Procurement Injury to the Graft
To increase the number of organs available, donation after cardiac death (DCD) is a new form of donation that is increasing in incidence (Chin et al, 2002). In this form of donation, a patient who is deemed hopeless but has not met criteria of brain death is allowed to die naturally after removal of supportive measures. After a period of usually 5 minutes of asystole, organs can be procured for transplantation. Despite the various periods of hypotension and warm ischemia that develop, outcomes with donation after cardiac death have been acceptable (Chin et al, 2002; Cooper et al, 2004). In liver grafts procured from DCD donors, increased biliary strictures and worse long-term graft survival have been suggested (D’Alessandro et al, 2004).
The most common injury during liver procurement is aberrant hepatic artery ligation and division. This injury occurs by failure to recognize a replaced or accessory right or left hepatic artery during hilar dissection or by unintentional division during organ removal. Such injuries are serious, because segments of the liver are not perfused during recovery and usually require reconstruction on the back table before reimplantation. An additional anastomosis on the back table prolongs ischemic time, increases the chances of thrombosis, and increases the need for retransplantation. Prolonged ischemic time, especially longer than 12 hours, increases the risk for graft loss and bile duct necrosis (Mor et al, 1993; Quiroga et al, 1991). To minimize dissection-related injuries, some authors prefer to use the en bloc method of removing abdominal organs, with back-table dissection (Imagawa et al, 1996). Regardless of the technique used, attention to detail and identification of the appropriate anatomy help avoid graft procurement injury.
Intraoperative Hemorrhage and Coagulopathy (See Chapter 70B)
Improvements in surgical techniques and better patient selection have led to the performance of liver transplantation without the need for blood transfusion in selected patients. The advent of the transjugular intrahepatic portosystemic shunt (TIPS) can significantly lower portal hypertension preoperatively and may help to reduce blood loss during transplantation (Forster et al, 1994); however, a misplaced TIPS in the vena cava or portal vein can be a life-threatening complication during liver transplantation. In these situations, TIPS removal is difficult and may lead to massive hemorrhage, if vascular control of the native vessels cannot be achieved.
In the presence of severe portal hypertension and underlying coagulopathy, the infusion of fresh frozen plasma and antifibrinolytic agents is the mainstay of therapy during liver transplantation (Palareti et al, 1991). These modalities cannot supplant the need for sound surgical technique with adequate control of all surgical bleeding sites. Bleeding observed after reperfusion may be related to poor initial graft function but also has been related to the release of heparin and heparin-like substances from the graft. Kettner and colleagues (1998) used heparinase-modified thromboelastography (TEG) to identify patients who developed bleeding secondary to the release of heparin-like substances. Such screening methods may help stratify patients at particular risk for the development of reperfusion fibrinolysis and may offer future therapeutic strategies to control coagulopathy encountered immediately after reperfusion (Kettner et al, 1998).
Primary Graft Dysfunction or Nonfunction
Numerous conditions can interfere with the initial function of the allograft after transplantation, including donor-related, procurement-related, and recipient-related factors. Donor-related factors that can affect graft function adversely include hemodynamic instability, poor nutritional status, extremes of age, drug toxicity, and steatosis (D’Alessandro et al, 2004; Marsman et al, 1996; Washburn et al, 1996).
Although no uniform definition exists, the severity and prognosis for graft dysfunction vary considerably. The most ominous syndrome is primary graft nonfunction, which usually requires immediate retransplantation. In such instances, a progressive increase is typically seen in serum transaminase levels (>8000 IU/L) within the first 24 to 48 hours in association with diminished bile and urine output. In our practice, postoperative trends in the prothrombin time (PT) have been the most reliable predictor of graft function and outcome. Laboratory studies drawn immediately after surgery serve as the baseline: the PT should plateau and then trend toward normalization in a matter of days with good graft function; any increase in the PT portends a worse prognosis and may represent primary graft nonfunction, especially if this occurs rapidly. An elevated PT that neither increases nor decreases may suggest primary graft dysfunction; recovery usually occurs with time, if no additional insults occur, such as infection or rejection. In these situations, infusion of prostaglandin E1 may be beneficial in resolving or preventing renal or graft dysfunction (Chavin et al, 1996; Klein et al, 1996).
In addition to trends in the PT, clinical assessment can be helpful in identifying patients with graft dysfunction or nonfunction. Resolution of hepatic encephalopathy, adequate urine production, and absence of metabolic acidosis are reassuring in the early postoperative period. In patients who have received significant quantities of blood products, the development of metabolic alkalosis may be a sensitive indicator of early graft function (Driscoll et al, 1987). Such indicators are based on the ability of the liver graft to process citrate in the administered blood products to bicarbonate; failure to metabolize citrate to bicarbonate may reflect early allograft dysfunction.
Vascular Complications
Hepatic Artery Thrombosis
Vascular complications are a major source of morbidity and graft loss in liver transplant patients. Arterial complications, of which hepatic arterial thrombosis (HAT) is the most common, account for 64% to 82% of the vascular complications encountered (Bell et al, 1990; Leonardi et al, 2004). The overall incidence of HAT is 1.6% to 8% in various adult series, but it can be 15% to 26% in pediatric patients (Busuttil et al, 1991; Mazzaferro et al, 1989; Tan et al, 1988). The incidence of HAT in LDLT varies widely and is influenced by the type of graft, surgeon experience, and donor anatomy.
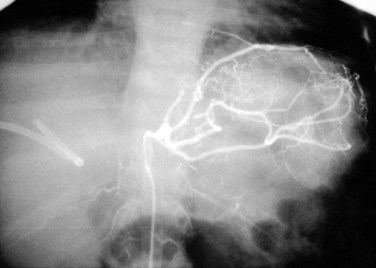
Doppler ultrasound (US) is the best screening method and should be used liberally in the first 2 weeks after transplantation with any change in graft function or significant elevation in bilirubin or transaminases. Because collateral blood flow through the gastroduodenal artery can result in a false-negative result, care must be taken to establish arterial flow within the hepatic parenchyma. In cases of suspected HAT revealed by US, confirmation should be made by celiac arteriography (Fig. 100.1) or multiphase computed tomography (CT). Failure to make a rapid diagnosis can result in hepatic necrosis and graft loss.
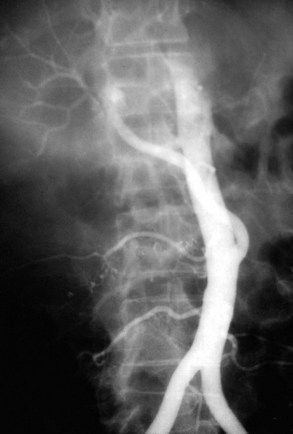
Intimal dissection of the recipient hepatic artery down to the origin of the celiac axis is a common cause of intraoperative HAT. In these cases, immediate reconstruction with a donor iliac allograft is indicated (Fig. 100.2). Under no circumstances should the patient leave the operating room without a completely revascularized graft. When a donor iliac allograft is unusable or unavailable, an autogenous saphenous vein graft should be used. Rarely, an artificial conduit made from Dacron or expanded polytetrafluoroethylene can be used.
The clinical presentation of HAT observed postoperatively ranges from a completely asymptomatic patient with minimal alterations in liver function to a critically ill patient with fulminant hepatic necrosis. A more common presentation of HAT is with postoperative biliary complications, including leaks and stricture formation (Margarit et al, 1998; Orons et al, 1995). Treatment for HAT in the early postoperative period is the same, whether symptoms are present or not: rapid reestablishment of arterial inflow should be attempted, which generally requires urgent operation with arterial reconstruction using a donor iliac artery allograft or autogenous graft material. Some authors have attempted thrombolysis using tissue plasminogen activator and urokinase, but this can be expected to be successful only when no technical factors are contributing to the thrombosis (Hidalgo et al, 1989). Hepatic artery intimal dissection is not amenable to thrombolytic therapy, and attempts at systemic thrombolysis waste valuable time and resources and worsen outcome. In most cases, 50% to 70% of patients ultimately require retransplantation (Langnas et al, 1991).
Portal Vein Thrombosis
Reconstitution of portal flow usually can be obtained through portal vein thrombectomy in most cases or by using a donor iliac vein allograft anastomosed between the superior mesenteric vein and liver allograft portal vein (Davidson et al, 1994; Shaked & Busuttil, 1991). Living-donor grafts that have relatively short portal vein segments can be difficult to reconstruct, and approximately 10% of institutions consider PVT an absolute contraindication to LDLT (Kadry et al, 2002).
PVT observed after transplantation is a rare complication that can occur in the immediate postoperative period, usually for technical reasons, such as incomplete thrombectomy or twisting of the anastomosis. PVT observed several months to years after transplantation usually results from intimal hyperplasia with gradual cavernous transformation with collaterals. A high index of suspicion is needed to make the diagnosis. Accumulation of ascites, splenomegaly, or the presence of varices after transplantation should prompt investigation. Early thrombosis is best treated with reoperation, thrombectomy, and systemic coagulation. The treatment of late thrombosis is more controversial, because direct repair is difficult. Transhepatic angioplasty with the placement of metal stents has been successful in some patients, whereas other patients have responded to selective shunting procedures to control variceal hemorrhage in the setting of adequate liver function (Jenkins et al, 1999; Raby et al, 1991).
Inferior Vena Cava Obstruction
Inferior vena cava (IVC) obstruction is a rare complication that occurs in 1% to 2% of patients after liver transplantation (Wozney et al, 1986). Most surgeons prefer to anastomose the donor IVC to the suprahepatic and infrahepatic IVC. A growing trend is to perform a so-called piggyback transplantation, with the anastomosis of the donor suprahepatic IVC to the confluence of the recipient middle and left hepatic veins, leaving the recipient IVC in situ (Neuhaus & Platz, 1994; Tzakis et al, 1989).
Treatment of an IVC thrombosis usually depends on the cause. Direct surgical removal is difficult in a critically ill patient and requires extensive mobilization of the right colon and small bowel mesentery to facilitate exposure. Medical management of IVC thrombosis was successful in our experience and that reported by others (Kraus et al, 1992). Our experience suggests that a more conservative management algorithm that employs invasive radiologic procedures and systemic anticoagulation can treat this complication satisfactorily (Kraus et al, 1992). Thrombolytic therapy can also be adjunctive in resolving “fresh” thrombus formation.
Biliary Complications
Biliary tract complications related to bile duct reconstruction previously were considered the Achilles heel of liver transplantation, but improvements in operative technique have reduced these complications markedly. Nevertheless, bile duct obstruction and leaks are the cause of approximately half of all technical failures after transplantation and require reoperation in 10% to 20% of patients (Lerut et al, 1987). In general, recipients of living-donor grafts have an incidence of biliary complications approximately two times higher than recipients of cadaveric grafts (Pomfret et al, 2001). HAT is associated with biliary complications and may explain the increased incidence among living-donor graft recipients.
Biliary Leaks
The reported incidence of biliary leaks after liver transplantation varies widely from 10% to 50% (Rabkin et al, 1998; Reichert et al, 1998). Leaks are observed most commonly at the site of choledochal anastomosis or the choledochal T-tube insertion site. Leaks at the choledochal tube insertion site are observed most commonly at the time of tube removal and occur in 25% to 40% of patients (O’Connor et al, 1995). Most of these patients can be managed conservatively with a short course of analgesics and antibiotics. To minimize this risk, some surgeons prefer not to place stents.
The bile ducts of living-donor or split-liver grafts can be reconstructed with a duct-to-duct (choledochocholedochostomy) or Roux-en-Y hepaticojejunostomy. The leak rate is similar with both types of reconstructions (Gondolesi et al, 2004). Signs and symptoms related to biliary leaks include bilious fluid in drains (biliary fistula), abdominal or shoulder pain or both, increased serum bilirubin, nausea, vomiting, and fever. Diagnosis can be confirmed by cholangiography if a choledochal tube is in place; otherwise, endoscopic retrograde cholangiography (ERC) or percutaneous transhepatic cholangiography (PTC) can be performed. We favor ERC, because treatment with endoscopic stent placement can be readily achieved without the risk associated with the indwelling transhepatic catheters used during PTC. Large biliary leaks resulting in bile collections adjacent to the liver require percutaneous drainage.
Biliary Stricture or Obstruction
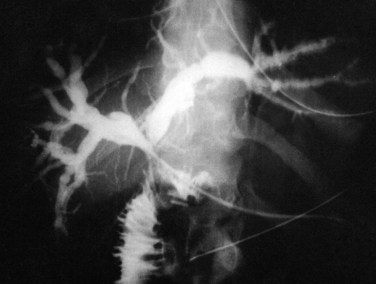
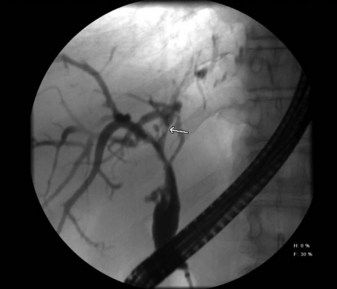
Biliary obstruction occurs in approximately 7% to 15% of patients after liver transplantation (Klein et al, 1991; Lerut et al, 1987). As with leaks, the site of obstruction aids in determining the cause. Anastomotic stricture accounts for 50% of obstruction cases and can occur early in the postoperative period, secondary to edema, or later, as a result of compromised blood supply (Fig. 100.3). Biliary strictures usually present within weeks but may occur years after transplantation. Two types of biliary obstruction usually are found: anastomotic and nonanastomotic. Nonanastomotic obstruction or stricture can be caused by or associated with bile duct ischemia or sludge and debris that can accumulate in the biliary system after transplanting (Fig. 100.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree