Chapter 42 Diagnostic and Operative Hysteroscopy: Polypectomy, Myomectomy, and Endometrial Ablation
INTRODUCTION
Hysteroscopy is an accurate surgical approach for evaluating the uterine cavity and treating a multitude of abnormalities. At its essence, it involves the transcervical placement of a lens and light system into the uterine cavity while using gas or liquid for distension of the cavity. Modern hysteroscopy is performed almost exclusively with the addition of a camera and video monitoring system. Operative hysteroscopy is performed using an electrosurgical resectoscope or a sleeve with an operating channel to accommodate instruments for grasping, biopsying, or cutting with scissors or laser.
HISTORY
Hysteroscopy, first described in the late 1800s, did not find widespread clinical use for more than 100 years. In 1869, Pantaleoni performed the first diagnostic and therapeutic hysteroscopy when he used a modified cystoscope to look into the uterine cavity and cauterize a hemorrhagic growth.1 Early hysteroscopists used a tube to mechanically distend the uterus for visualization. Near the beginning of the 20th century, Dr. Isidor C. Rubin, a New York gynecologist best remembered for Rubin’s test, first used carbon dioxide (CO2) to distend the uterus for hysteroscopy. Around the same time, Professor C. J. Gauss, a German surgeon and descendant of the famous mathematician, first performed hysteroscopy using fluid distension media.2 However, hysteroscopy did not find widespread acceptance for another half-century.3
In the 1970s interest in hysteroscopy renewed, in parallel with the rapid advancement of diagnostic and operative laparoscopy. Probably the most important advances came in the form of improved methods for distending the uterine cavity, including use of viscous and low-density liquid solutions. Around the same time insufflation machines were designed for CO2 and fluid media that utilized high pressure and low flow, rather than the low pressure and high flow used for laparoscopy.4–6 Carbon dioxide was often used for diagnostic hysteroscopy, and fluid media became the standard for operative hysteroscopy. It was found that isotonic fluid was ideal for most operative procedures, whereas nonconductive hypotonic media was required for electrosurgical procedures.
The use of hysteroscopy became widespread in the 1980s with the development of better optics and lighting and the use of video cameras. Operative techniques for various intrauterine pathologic conditions continued to be developed. Most notably among these may be removal of submucous myomas using an enhanced urologic resectoscope,7 removal of uterine septum,8 and endometrial ablation using laser,9 resectoscopic loop,10 or rollerball.11 Today, hysteroscopy has become a standard diagnostic and therapeutic technique performed by most gynecologists.
INDICATIONS
Operative Hysteroscopy
The two most common indications for operative hysteroscopy are (1) directed biopsy of focal lesions and (2) treatment of intrauterine lesions, including endometrial polyps and intracavitary leiomyomas, uterine septa, and cornual tubal occlusion. Removal of uterine septum and treatment of cornual occlusion with cannulation are covered in Chapters 43 and 47.
Less common indications for operative hysteroscopy include retrieval of a “lost” intrauterine device with a retracted tailstring, discussed in Chapter 27. Hysteroscopy has also been used in early pregnancy for chorionic villus sampling. The newest indication is the placement of coils into the intramural section of the tube for permanent sterilization, which is covered in Chapter 28.
BASIC HYSTEROSCOPIC EQUIPMENT AND TECHNIQUES
Distension Media
Carbon Dioxide
Another problem associated with the use of CO2 for hysteroscopy is the rare occurrence of fatal gas embolism. Although a small amount of CO2 is rapidly absorbed and cleared from the body via respiration, an open vessel in the uterus can allow enough gas into the venous system to result in fatal heart block.12 To minimize this risk when CO2 is used for hysteroscopy, it has been recommended that the Trendelenburg position should be avoided, the cervix should not be dilated, and no operative procedures should be performed.
Dextran 70
Dextran 70 is rarely used today as hysteroscopic distension media because of several associated problems.13 From an operational perspective, dextran 70 solutions cause instruments such as graspers and scissors to become permanently inoperable if the solution is allowed to dry on the instruments. This problem can usually be avoided by immediate cleaning shortly after finishing the procedure.
Finally, allergic reactions to dextran 70 have also been reported. The risk of anaphylaxis while using dextran 70 for hysteroscopy has been estimated to be as high as 1 per 1500 cases.14
Low-Viscosity Fluid
Hypotonic nonelectrolyte-containing fluids are required when the unipolar resectoscope is used, and several types are available. The most common fluids used are 5% mannitol, 3% sorbitol, and 1.5% glycine. The theoretical advantage of 5% mannitol is that it is rapidly broken down by the liver into glycogen and is excreted through the kidney, with a half-life of 100 minutes.15
When a fluid deficit of 1,000mL of nonelectrolyte solution is identified, blood should be drawn to determine electrolyte levels, the procedure should be terminated, and consideration should be given to administering diuretics, with close monitoring of electrolytes. Injection of 3 to 4mL of dilute vasopressin (10 units in 50mL saline solution) into the cervix decreases both intraoperative bleeding and intravasation for at least 20 to 30 minutes.16
DIAGNOSTIC HYSTEROSCOPY
In the 1980s, the development of hysteroscopes with smaller diameters (<4mm) allowed the use of the hysteroscope for diagnosis without the need for either cervical dilation or anaesthesia. As a result, office hysteroscopy has become a common procedure, which has been documented to have the advantages of patient acceptability, diagnostic accuracy, and cost- effectiveness.17 Hysteroscopy is particularly useful for identifying focal lesions, which are often missed with endometrial sampling.18
Office Hysteroscopy
A low (<1%) complication rate is noted when office hysteroscopy is performed by skilled physicians. Complications include uterine perforation, infections, excessive bleeding, and complications related to the distension media.19
Technique
A slow and thorough evaluation is important to identify abnormalities of the endocervix, fundus, and tubal ostia. Myomas of the endocervix and lower uterine segment can easily be missed with too rapid insertion of the hysteroscope. Likewise, if the hysteroscope is too quickly placed above an intracavitary lesion, it can likewise be missed.
Lesion Size
The size of lesions cannot be accurately determined hysteroscopically, in contrast to transvaginal ultrasound. This is because the hysteroscopic eyepiece is focused at infinity, thereby making the objects that are closer appear magnified and objects viewed further away smaller.20 This phenomenon can lead to surprises in the operating room, especially when the size of a lesion, particularly submucosal myomas, may be underestimated.
HYSTEROSCOPIC POLYPECTOMY
Polyps are often symptomatic. However, in women with abnormal uterine bleeding, investigation may lead to their detection. Symptoms most often related to uterine polyps include abnormal bleeding, postcoital staining, chronic vaginal discharge, or dysmenorrhea. Polyp-related bleeding is often characterized by increased clotting, intermenstrual or premenstrual spotting, or heavier menstrual flow. There is good evidence that polyps can decrease fertility and that their removal will improve the chances of pregnancy.21
It is obvious that symptomatic endometrial polyps should be removed. However, it is also important to remove asymptomatic polyps, particularly in postmenopausal women.22 Although the vast majority are benign, endometrial cancer and hyperplasia will be found in approximately 2% of endometrial polyps and are associated with coexisting malignancies elsewhere in the endometrium. In one study of more than 1400 polyps, endometrial cancer was found in 27 polyps (1.8%).22 All but one of these women were postmenopausal, and 26% were asymptomatic.
HYSTEROSCOPIC MYOMECTOMY
Leiomyomas are the primary indication for more than 40% of the 650,000 hysterectomies performed annually in the United States.23 Submucosal myomas likely account for 10% to 20% of all myomas. Many of these can be removed by operative hysteroscopy. In addition to preserving fertility in many cases, a hysteroscopic approach is associated with a shorter recovery period, lower complication rate, and lower cost than hysterectomy.
For a successful surgical outcome, it is important to identify preoperatively the size, number, location, and depth of intramural extension of uterine myoma. Myoma size, number, and location are determinants of complete resectability, the number of surgical procedures necessary for complete resection, the duration of surgery, and the potential complications from fluid overload.24
Numerous studies have demonstrated that preoperative saline infusion sonohysterography gives more information than hysteroscopy in respect of myomas. Chapter 30 reviews the topic of ultrasonography and sonohysterography in detail.
Hysteroscopic Classification of Myomas
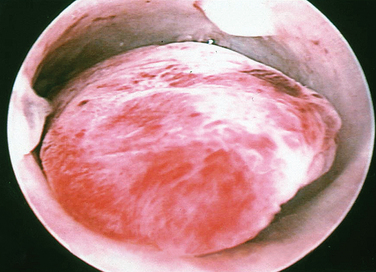
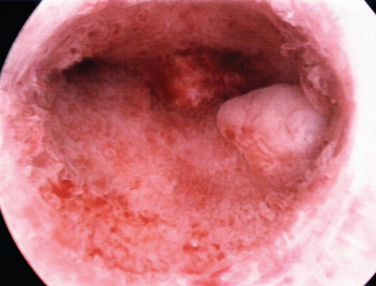
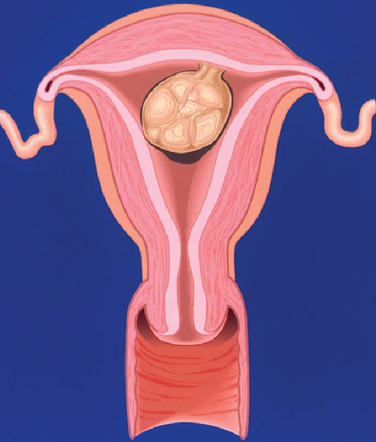
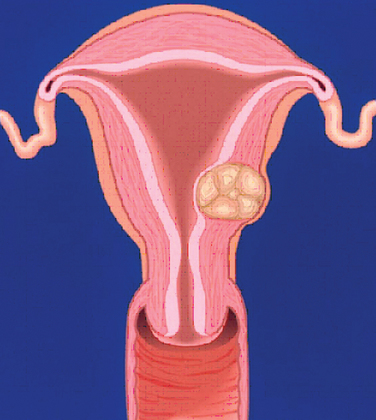
A classification system is important to most accurately determine the appropriate method for performing myomectomy and counseling the patient on risk and prognosis. The European Society of Hysteroscopy classification system is based on myoma location and the amount of myoma protruding or encroaching on the endometrial cavity.25 In this system, Type 0 myomas are pedunculated, with the myoma lying completely within the endometrial cavity (Fig. 42-1). Type I myomas are described as sessile, with less than 50% intramural extension (Fig. 42-2). Finally, Type II myomas are submucosal in location, with more than 50% intramural extension. These include transmural myomas, which extend from the submucosal to the serosal edge. When viewed hysteroscopically, Type II myomas appear as a “bulge” into the endometrial cavity. Multiple myomas such as those in Figure 42-3 are not placed within this classification. They should not accessed by hysteroscopy.
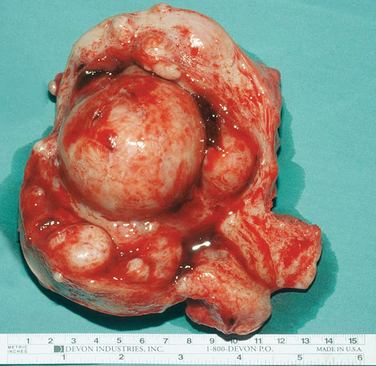
Figure 42-3 Extirpated uterus showing a large (6 cm) intracavitary myoma with multiple intramural and submucous myomas.
This system was originally designed to classify myomas exclusively on hysteroscopic appearance. However, this approach has significant limitations. During hysteroscopy, myomas can be compressed and recede into the myometrium as a result of the pressure of the distension media, thereby preventing full visualization of the myoma. For this reason, preoperative evaluation with ultrasonography is required to accurately determine how many myomas are present and how deeply the myomas penetrate the myometrium.
An ultrasonographic classification system has been developed for intramural myomas that corresponds in part to the hysteroscopic classification and in part to the hysterosalpingography data26 (Table 42-1).
Table 42-1 Hysteroscopic and Sonohysterographic Classification System for Myomas Encroaching Upon the Endometrial Cavity
| Hysteroscopic Type25 | Sonohysterographic Class26 | Description |
|---|---|---|
| Type 0 | Class 1 | Pedunculated myomas, where 100% of the myoma lies within the endometrial cavity with no intramural extension |
| Type I | Class 2 | Sessile myomas, with <50% intramural extension |
| Type II | Class 3 | Submucous myomas, with >50% intramural extension |
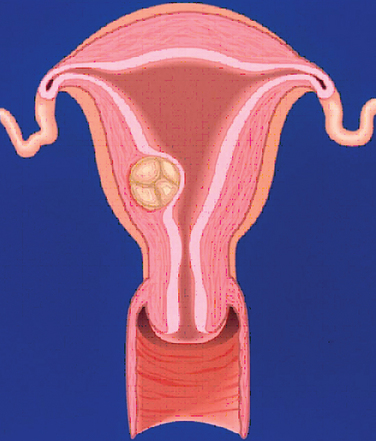
Figure 42-5 Class 2 myomas have a submucosal component that involves less than 50% of the myometrium.
Surgical Approach According to Stage
The degree of surgical difficulty and thus the risk to the patient is related to the depth of penetration and size of the myomas. Pedunculated hysteroscopic Type 0 or sonohysterographic Class 1 myomas up to 3cm in dimeter can usually be easily removed hysteroscopically. Larger hysteroscopic Type 0 myomas (>3cm) and hysteroscopic Type I (Class 2 on sonohysterography) myomas can be approached hysteroscopically. However, the risk of fluid intravasation increases as a result of increased surgical time and the opening of myometrial venous channels during resection. Operative hysteroscopy is made more difficult by limited space within the uterus and poor visibility due to an inability to further distend the uterine media and the large amount of myoma “chips” that accumulate within the endometrial cavity. Often, incomplete removal of larger myomas requires two or more separate operative procedures. Only the most experienced hysteroscopist would attempt a hysteroscopic resection of an intracavitary myoma 5cm or larger. Myomas that are large and multiple (see Fig. 42-3) should not be excised by hysteroscopy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree