Chapter 14 Reproductive Disorders in the Adolescent Patient
MENSTRUAL DISORDERS
Dysmenorrhea
In adolescents, the incidence of dysmenorrhea can be quite high and is often underreported or ignored by healthcare providers.1–3 In a survey of 2699 normal adolescents, the prevalence of dysmenorrhea was 59.7%.2 The authors noted that the prevalence of dysmenorrhea increased with age from 39% at age 12 to 72% at age 17. In a large Swedish epidemiologic study on adolescents with dysmenorrhea, 15% of responders reported disrupted daily activity secondary to severe dysmenorrhea.1
Primary and secondary dysmenorrhea must be differentiated because management may vary significantly (Table 14-1). Primary dysmenorrhea is defined as menstrual pain that is not associated with a specific underlying pathology. Secondary dysmenorrhea is menstrual-associated pain that is due to an underlying pathologic process. History and pelvic examination are used to rule out secondary dysmenorrhea. Primary dysmenorrhea requires the initiation of ovulatory cycles. Thus, primary dysmenorrhea will present typically within the first 12 to 24 months after menarche.
Table 14-1 The Gynecologic Differential Diagnosis of Pain with Menses
| Primary | Prostaglandin production abnormalities |
| Secondary |
The onset, duration, and intensity of pain should be recorded. Cramps commonly begin on the first day of menses. The median duration of dysmenorrhea is 2 days.4 Typically, pain diminishes after the third day of bleeding. The patient may report associated symptoms of nausea, vomiting, low back pain, headache, diarrhea, dizziness, and occasionally fainting.5
The severity of dysmenorrhea appears to increase with the duration of menstruation.6 Certain features of the personal history may point the investigation in a particular direction. For example, a family history of endometriosis may increase the level of suspicion for this disease. The onset of pain with menarche raises the possibility of an obstructive müllerian anomaly.
Pathogenesis of Dysmenorrhea
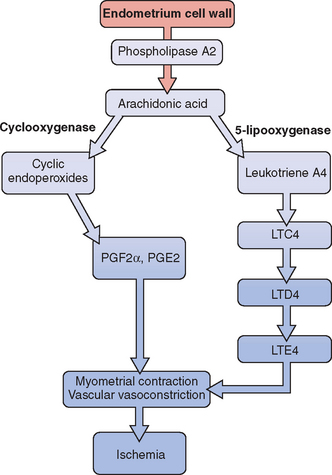
Release of prostaglandin F2α (PGF2α) from the secretory endometrium leads to myometrial contractions and subsequently primary dysmenorrhea (Fig. 14-1). Multiple studies have documented increased levels of PGF2α in the menstrual fluid and endometrial tissue of women who are dysmenorrheic versus eumenorrheic women.7,8 Release of prostaglandins into the circulation, typically within the first 48 hours from initiation of menses, accounts for some of the associated symptoms of nausea, headache, vomiting, and diarrhea.
The uterus can produce and metabolize leukotrienes (LT). Higher leukotriene levels are present in the myometrium and endometrium of adult women with dysmenorrhea.9 Levels of LTC4 and LTD4 have been found to be significantly higher in the menstrual blood of women with primary dysmenorrhea than in those without.10 Thus, these potent vasoconstrictors and inflammatory mediators may play a role in producing the symptom of dysmenorrhea.
Treatment
Many adolescents self-medicate, but it is typically in subtherapeutic doses. Lower doses of over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) have been used in conjunction with other modes of treatment such as local heat therapy, exercise, and herbal therapy. NSAIDs reduce prostaglandin production by inhibiting cyclo-oxygenase. Multiple studies have shown that NSAIDs reduce the severity of symptoms associated with dysmenorrhea.11 A clear advantage of one NSAID over another has not been demonstrated.
If NSAIDs are unsuccessful in the management of symptoms, oral contraceptives are recommended as the next tier of therapy. By decreasing endometrial growth, oral contraceptives limit the production of prostaglandins and leukotrienes. Indeed, lower levels of prostaglandins have been reported in the menstrual fluid of women on oral contraceptives compared with patients with dysmenorrhea who were not taking these drugs.7
There is clear evidence that adolescents taking oral contraceptives have reduced rates of dysmenorrhea.12 Because depot medroxyprogesterone acetate (DMPA) inhibits ovulation and causes endometrial atrophy, it may be used to decrease dysmenorrhea symptoms in adolescents. However, the possible side effects—including osteoporosis—may limit its long-term use in adolescents.
Dietary changes may impact dysmenorrhea. In a prospective, randomized, double-blind crossover study, 42 adolescents between the ages of 15 and 18 were given placebo or fish oil for 2 months.13 The results showed a significant reduction in dysmenorrhea in those who received the fish oil supplementation. This study seems to suggest that supplementation with omega-3 fatty acids may alleviate dysmenorrhea in adolescents. It has been suggested that omega-3 fatty acids prevent buildup of the more potent prostaglandins and leukotrienes that are associated with vasoconstriction and myometrial contractions.
Polycystic Ovary Syndrome
Irregular menstrual cycles, an important characteristic of polycystic ovary syndrome (PCOS), are frequently encountered during the first couple years of menstruation.14 Most of these menstrual abnormalities normalize in the first 2 years after menarche, but careful follow-up and examination are necessary. In one study, half of adolescent girls between the ages of 14 and 16 with oligomenorrhea still had this menstrual problem at age 18.15
Polycystic ovary syndrome is the most common reproductive endocrine abnormality associated with irregular periods in adult women of reproductive age. Because this syndrome starts at puberty, adolescents must be monitored closely for signs of this disorder because early intervention may have a beneficial long-term effect.16 Although subtle signs and symptoms may exist during childhood, the diagnosis is typically deferred until puberty when patients present with irregular menstruation. During the peripubertal period, the hypothalamic-pituitary-ovarian (HPO) axis is relatively immature, which can lead to anovulatory cycles and make it difficult to diagnose PCOS during this time.
Menarche usually occurs at a normal age in adolescents with PCOS. But data suggest that premature pubarche may be associated with development of PCOS.17–19 In a group of postpubertal adolescents with premature pubarche, 45% of the patients showed evidence of functional ovarian hyperandrogenism.17 In a study comparing 98 children with premature pubarche to Tanner stage and bone age-matched controls, mean serum insulin levels were elevated in all participants with premature pubarche for all Tanner stages. Hyperinsulinemia was noted in all participants with premature pubarche, implying that insulin resistance was present during childhood.20
Signs and Symptoms
According to the Rotterdam European Society of Human Reproduction (ESHRE)/American Society for Reproductive Medicine (ASRM)-sponsored PCOS consensus workshop, two of the following three criteria must be present in order to diagnose PCOS: irregular periods, polycystic ovaries on ultrasound, and physical or biochemical evidence of excess androgens.16 Other etiologies, such as late-onset congenital adrenal hyperplasia (CAH), hyperprolactinemia, or androgen-secreting tumors, which may lead to hyperandrogenism, must be excluded.
The most common presenting complaint of adolescents with PCOS is oligomenorrhea followed by episodes of menorrhagia. The presence of oligomenorrhea at age 15 was shown to be a better predictor of oligomenorrhea at age 18 than high testosterone levels, luteinizing hormone (LH) concentration, or polycystic ovaries at ultrasound.14
Hirsutism in adolescents is most commonly caused by PCOS.21 Other skin manifestations of hyperandrogenism include acne and alopecia. Acanthosis nigricans and skin tags may be seen secondary to the accompanying hyperinsulinemia. Girls with PCOS had higher LH and androgen concentrations than controls.22 The same findings have been reported in women with PCOS. Increased LH pulsatility, elevated LH/follicle stimulating hormone (FSH) ratios, and increased ovarian volume have been described in girls with PCOS.23
During childhood, obesity is the prime cause of insulin resistance.24 However, PCOS in adolescents (and women as well) bestows a risk for hyperinsulinemia independent of weight and body composition. Euglycemic clamp studies in two groups of obese 12-year-old girls with and without PCOS showed that fasting plasma glucose levels in the PCOS group were higher than those in the non-PCOS group. Insulin sensitivity was decreased by 50% in the PCOS group compared with the non-PCOS group.25 This metabolic abnormality is a precursor to type 2 diabetes mellitus and is generally detected early in the course of PCOS. Indeed, a 33% rate of impaired glucose tolerance is noted in obese adolescents with PCOS.26 Hyperinsulinemia has also been detected in lean oligomenorrheic and hyperandrogenic adolescents.27 Thus, lean PCOS adolescents are also at increased risk of developing type 2 diabetes mellitus.
Depression has been detected in one half of women with PCOS. The symptoms of hirsutism, obesity, acne, and alopecia place significant emotional stress on the adolescent during a time when she is particularly vulnerable. Because social acceptance is so important during adolescence, the girl who physically looks different from her peers is more likely to feel social anxiety.28–30
It is important to obtain a complete family history of significant endocrine problems. A family history of PCOS appears to be a significant risk factor for the development of PCOS. The rates of PCOS in premenopausal mothers and sisters of patients with PCOS are 35% and 40%, respectively.31 The patient is questioned regarding the existence of PCOS, male alopecia, female hyperandrogenism, menstrual irregularities, diabetes, obesity, and infertility on either her mother’s or father’s side of the family.
Laboratory Evaluation
Endocrinologic evaluation in the adolescent is the same as that in adults and includes assessment of serum androgens as well as exclusion of other endocrine disorders associated with similar presentations such as late-onset CAH and hyperprolactinemia. Details of this investigation are discussed in detail in Chapter 15. Periodically, an oral glucose tolerance test may be necessary to assess for impaired glucose tolerance—especially in obese adolescents. Although ultrasound of the pelvis may be performed instead of a pelvic examination in the apprehensive young patient, it is not absolutely necessary.
Treatment
Oral contraceptives not only regulate menses, but also decrease hirsutism and acne. By inhibiting gonadotropin secretion and increasing sex hormone-binding globulin, oral contraceptives decrease the amount of free testosterone, with a subsequent beneficial effect on the hair follicle. Contrary to what most adolescents believe, oral contraceptives do not increase body weight or fat in teenagers with PCOS.32 However, there is some concern that they may increase insulin resistance in a group already at risk for hyperinsulinemia.33
Antiandrogens such as spironolactone are quite effective in diminishing the symptoms of hirsutism and acne. A decrease in the width of the hair shaft and amount of sexual hair growth is noted at doses up to 200 mg/day. Menstrual abnormalities may occur with use of spironolactone; thus, concomitant use of oral contraceptives is commonly recommended. Hair growth changes may not be evident for 6 to 9 months. But once the rate of hair growth is decreased, she may proceed with use of cosmetic agents such as electrolysis and laser therapy. Eflornithine cream, which inhibits ornithine decarboxylase—an enzyme within the hair follicle—can slow hair growth.34 It may be applied twice a day to the affected area, but as with spironolactone, it may take several months to see any improvement in hair growth.
The role of insulin-sensitizing agents in the management of PCOS in adolescents is not clear. Like adults, many PCOS adolescents have insulin resistance and are at increased risk for developing diabetes and cardiovascular disease. But the first line of therapy to normalize the abnormal metabolic profile of such patients is lifestyle modification, which includes exercise and diet. Lifestyle modification in adolescent girls is especially important because behavioral changes during puberty may have long-lasting effects. Lifestyle modification may be even more beneficial than metformin therapy in the prevention of diabetes.35 In addition, data documenting the safety of metformin use and long-term outcome in adolescents are lacking. Thus, it may be difficult to justify lifelong use of metformin in this population.
There is good evidence, however, that metformin can normalize abnormal metabolic parameters both in obese and nonobese adolescents. A study of 15 obese adolescents with PCOS and impaired glucose tolerance who were given metformin 850 mg twice a day showed that the therapy decreased hepatic and peripheral insulin resistance, improved glucose tolerance, and decreased free and total testosterone levels.36 When metformin was given to nonobese adolescents, an improvement in hirsutism, free androgen index, lipid profile, and insulin parameters was again noted.37 Unfortunately, all improvements in lipid profile, hyperandrogenism, menstruation, and hyperinsulinemia are reversed within 3 months of termination of metformin.37,38
Abnormal Uterine Bleeding
Abnormal uterine bleeding (AUB) is very common in girls for the first 1 to 2 years after menarche. It is defined as excessive, prolonged, or irregular bleeding in the absence of structural lesions of the uterus. In a study evaluating the menses of over 5000 adolescents, the incidence of irregular menstrual cycles was 43% during the first year of menses and 20% in the sixth year.3 Thus, most adolescents eventually experience normal menses, but in the longest follow-up study on record, 5% continued to have severe episodes of anovulatory bleeding in adult life.39
Etiology
Because AUB is a diagnosis of exclusion, other possible causes of vaginal bleeding must be considered. The differential diagnosis of AUB is vast; a summary is given in Table 14-2. A more detailed review is given in Chapter 21. In most instances, the cause of anovulation remains unknown in adolescents. Chronic illnesses such as liver cirrhosis and renal failure may contribute to anovulation and subsequent AUB. Other circumstances that can lead to anovulation include eating disorders, weight changes, and vigorous athletics. Because it may be difficult to obtain a correct sexual history from a teenager, pregnancy complications such as a missed abortion must be considered. Although local disorders such as leiomyomas are uncommon, others such as cervicitis are more common and present as prolonged vaginal bleeding.
Table 14-2 Differential Diagnosis of Abnormal Uterine Bleeding
| Pregnancy-related disorders |
| Disorders of blood hemostasis |
| Chronic illness |
| Sexually transmitted disease |
| Endocrine disorders |
| Medication |
| Local gynecologic abnormalities |
Teenagers presenting with ovulatory AUB (menorrhagia) must be assessed for bleeding disorders. In one retrospective study, a primary coagulation defect was found in 19% of patients who were admitted to the hospital with acute menorrhagia. The prevalence of bleeding disorders rose to 50% in the subgroup of girls with menarchal menorrhagia.40 More recent studies in which the bleeding disorder was more likely to have been detected during premenarche showed a much lower rate of newly diagnosed coagulopathy.41,42 Only 3% of patients admitted for menorrhagia in these studies had a newly diagnosed coagulation disorder. On the other hand, patients hospitalized with hematologic disorders that require chemotherapy may develop severe vaginal bleeding.
Evaluation
The laboratory evaluation in teens with excess vaginal bleeding initially includes a pregnancy test and complete blood count with platelets. The complete blood count can be used to evaluate the severity of the bleeding and as a guideline to determine the urgency of care. In addition, it may help rule out some major hematologic problems. If a bleeding disorder is suspected, a bleeding time, a prothrombin time, and activated partial thromboplastin time as well as a screening test for Von Willebrand disease should be ordered. Details of these are given in Chapter 21. Patients should be evaluated for potential endocrine disorders such as thyroid disease and pituitary adenomas.
Treatment
There are data to support the use of NSAIDs to decrease the amount of blood loss.43,44 This is especially the case in patients with ovulatory AUB (menorrhagia). Patients presenting with mild anemia frequently experience moderate to heavy menses every 2 to 3 weeks. In such cases, oral contraceptives or progesterone may be used to control the bleeding. Use of medroxyprogesterone acetate or norethindrone acetate for 10 to 14 days can help stabilize the endometrium followed by global shedding and controlled period. These agents may not be successful in cases where the bleeding has been heavy and prolonged. In such cases, the endometrium usually is very thin and some amount of estrogen is necessary to heal the lining. Thus, monophasic combined oral contraceptives may be more effective. Although 10 to 14 days of cyclic progestin therapy is adequate for anovulatory AUB, luteal phase progestin therapy is less effective for ovulatory AUB. Progestin therapy for 21 days of the cycle (day 5 to day 26, for example) is more effective.
Treatment of Severe Anemia
Adolescents whose initial hemoglobin level is less than 7 g/dL and are bleeding actively and heavily may need to be admitted for blood transfusion. A coagulation panel must be assessed prior to any blood transfusion or hormonal manipulation. If deemed clinically stable, she may be initiated on oral contraceptives. However, if she is severely hemorrhaging or unable to take medications orally, she can be given intravenous conjugated estrogens (Premarin) 25 mg every 4 hours for 24 hours or less if she stops bleeding.45 Once the bleeding has decreased or stopped, the patient should be given a combination oral contraceptive or progesterone.
In instances where high-dose estrogen therapy is contraindicated, high doses of progesterone such as norethindrone and medroxyprogesterone acetate may be used. Gonadotropin-releasing hormone agonists have also been used to manage AUB in patients who are unresponsive to hormonal management or have contraindications to their use. Antifibrinolytic agents have been used successfully to manage menorrhagia. However, there is minimal data on their use for this indication in adolescents. In one comparative study in adults, mefenamic acid decreased blood loss by 20% and tranexamic acid reduced blood loss by 54%.46 Its use in adolescents has been reserved for patients with organic pathology in whom hormonal therapy has failed.
Dilatation and curettage and hysteroscopy are indicated only when the patient is unresponsive to medical therapy—they are the last line of evaluation and therapy for teenagers. A report of successful endometrial ablation on a teen with severe uterine bleeding complicated by end-stage renal disease exemplifies the critical nature of some patients.47 However, this procedure is only considered an alternative to hysterectomy. Other medical treatments that are used in adults have been sporadically used in adolescent girls (see Chapter 21).
Long Term Outcome
Continued follow-up is mandatory in adolescents experiencing AUB, as illustrated by the results of a 25-year follow-up study.39 This study was performed before hormonal therapy was an option. However, it is quite revealing that the change to normal menses was greatest in the first 2 years of the study. By 4 years, 50% still had AUB. No patient developed normal periods after 10 years of persistent abnormal bleeding. More than half of these patients presented with infertility. Hence, follow-up provides an opportunity to either reassure the adolescent as she normalizes or to educate her about the need for continued medical therapy and potential options for the future.
AMENORRHEA
Primary amenorrhea is defined as the absence of any menstrual flow by age 15 if the patient has developed secondary sexual characteristics or within 5 years of breast development. Secondary amenorrhea is defined as the absence of menstruation for 6 months in an adolescent who has previously established menstruation or the absence of menstruation for a minimum of 3 previous cycle intervals. Although these terms provide information regarding the menstrual cycle, they do not help exclude various diagnoses. Amenorrhea is discussed in detail in Chapter 16. However, an overview is given here to address the differential diagnosis in an adolescent girl who has gone through puberty normally but presents without a period. These patients typically enter the healthcare system through a gynecologist. Patients may have amenorrhea associated with a pubertal disorder. This topic is covered in a separate chapter (see Chapter 11).
Amenorrhea without Pubertal Delay
The differential diagnosis of the adolescent presenting with normal secondary sexual characteristics and amenorrhea is vast (Table 14-3). Hence, only the most common ones are discussed here. Pregnancy must be excluded first. The adolescent is questioned and assessed for signs of hirsutism, galactorrhea, weight changes, dietary changes, changes in exercise patterns, chronic illness, visual changes, and headaches.
Table 14-3 Differential Diagnosis of Amenorrhea without Pubertal Delay
| Congenital anomalies of the genital tract | |
| Specific gonadal disorders | |
| Hypothalamic/pituitary disorders |
Imperforate Hymen
This anomaly results from incomplete canalization of the hymen (Fig. 14-2). It is most commonly detected during adolescence. It may be diagnosed in utero or during the neonatal period.48 The incidence of this anomaly is approximately 0.1% of term infant girls, and it is believed to occur sporadically. However, there have been rare reports of familial occurrence of imperforate hymen.49,50 Although a common mode of inheritance has not been identified, it seems prudent to obtain a family history of reproductive tract defects.
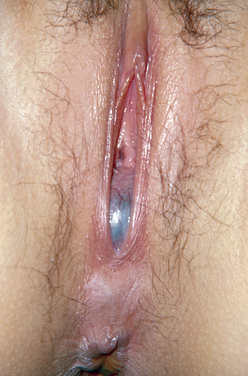
Figure 14-2 In this photograph, a bulging bluish membrane is easily seen that represents an imperforate hymen.
When detected during infancy, the imperforate hymen presents as a mucocele. It is typically asymptomatic and evident on inspection of the external genitalia. In some instances, the hydrocolpos is large enough to cause urinary tract obstruction and urine retention. In such cases, surgical decompression of the hydrocolpos is necessary. Urinary tract infections have been reported in patients with mucoceles and may potentially lead to formation of a pyocolpos.51
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree