16
Cystic and neuroendocrine tumours of the pancreas
Introduction
Although pancreatic ductal adenocarcinoma accounts for the majority of patients with neoplastic disease of the pancreas, over the last two decades there has been an increasing recognition of cystic and neuroendocrine pancreatic neoplasms.1 The aim of this chapter is to examine these tumours in more detail, with particular emphasis on intraductal papillary mucinous neoplasms (IPMNs) and pancreatic neuroendocrine tumours (NETs). Where possible, evidence-based recommendations for the investigation and management of these tumours will be provided.
Intraductal papillary mucinous neoplasms
IPMNs have only been recognised as separate entities to ductal adenocarcinoma of the pancreas since 1982,2 subsequent to which the World Health Organisation clarified their definition.3 They are defined as a grossly visible, mucin-producing epithelial neoplasm of the pancreas, which arises from within the main pancreatic duct (main-duct IPMN) or one of its branches (branch-duct IPMN), and most often but not always has a papillary architecture. They are distinguished from mucinous cystic neoplasms (MCNs) by the absence of ovarian-type stroma.4
The incidence (95% confidence interval) is estimated at 2.04 (1.28–2.80) per 100 000 population; however, this increases significantly after the sixth decade.5 The precise aetiology remains unknown, although an association with extrapancreatic primaries (10%), most commonly colorectal, breast and prostate, has been reported, but this is not significantly different to that seen with primary pancreatic adenocarcinoma.6 IPMN has also been shown to be a predictor of pancreatic cancer as compared to other intra-abdominal pathologies, with an odds ratio of 7.18.7
Clinical presentation
IPMNs most commonly present with symptoms related to pancreatic duct obstruction. The Johns Hopkins group reported their experience comparing the presentation and demographics to those patients presenting with pancreatic adenocarcinoma.8,9 Although the mean age of presentation was similar to that of pancreatic adenocarcinoma (seventh decade), the clinical presentation was significantly different. Of the 60 patients with IPMNs, 59% presented with abdominal pain but only 16% presented with obstructive jaundice, compared to 38% and 74% of patients with pancreatic adenocarcinoma, respectively.8 This is in spite of the fact that only five of the 60 patients with IPMNs had tumours within the body or tail.8 In addition, those with IPMNs were more likely to have been smokers and 14% had suffered previous attacks of acute pancreatitis (compared to 3% of those with pancreatic ductal adenocarcinoma).8 Weight loss was a prominent factor reported in 29% of patients with IPMNs.9 Symptoms associated with invasive malignancy included the presence of jaundice, weight loss, vomiting9 and diabetes.10 Patients with invasive IPMNs were a mean of 5 years older (68 vs. 63 years) compared to those with non-invasive IPMNs.9 This led the authors to conclude that IPMN was a slow-growing tumour with a significant latency to develop invasive disease.9 Increasingly, an important presentation is the incidental finding due to cross-sectional imaging for other medical indications. IPMN was the final diagnosis in 36% of pancreatic ‘incidentalomas’ that underwent pancreatico-duodenectomy.11
Investigation
Computed tomography (CT) and magnetic resonance imaging (MRI) form the mainstay of non-invasive radiological imaging of suspected IPMN. The classical features of main-duct IPMN are of a grossly dilated main pancreatic duct (Fig. 16.1), while branch-type IPMN can present with small cystic lesions that may appear in a ‘grape-like’ configuration.12 Although MRI and CT have been shown to identify accurately tumour location and communication with the pancreatic duct, the detection of invasive malignancy remains problematic.13–15 Radiological features associated with malignancy include the presence of a solid mass, biliary dilatation > 15 mm and increasing size of either the tumour for branch-type IPMN (growth rate > 2 mm/year)16 or main pancreatic duct diameter for main-duct IPMN.15 18F-labelled fluorodeoxyglucose CT/positron emission tomography (PET) has recently been shown in small case series to differentiate benign from malignant IPMNs. In a series of 29 patients, a standardised uptake value of > 2.5 was shown to have a 96% accuracy in determining the presence of malignancy.17 Differentiating IPMN from other cystic neoplasms (particularly branch-type IPMN from MCN) can be difficult and the importance of considering the clinical picture cannot be underestimated, particularly the patient’s age, gender and history of pancreatitis or genetic syndromes.18 Radiologically, localisation within the uncinate process, detection of non-gravity-dependent luminal filling defects (papillary projections) or grouped gravity-dependent luminal filling defects (mucin), and upstream dilatation of ducts (MCN ducts are normal) all favour the diagnosis of branch-type IPMN.19 Differentiating diffuse main-duct IPMN from chronic obstructive pancreatitis can be challenging radiologically19 (clinically, patients with IPMN tend to be 20 years older and lack a history of heavy alcohol use), but high-quality cross-sectional imaging looking for endoluminal filling defects (either mucin or papillary proliferations), cystic dilatation of collateral branches (particularly within the uncinate process), communication of dilated ducts with normal ducts without evidence of an obstructing lesion or a widely open papilla (Fig. 16.1) all favour IPMN.19
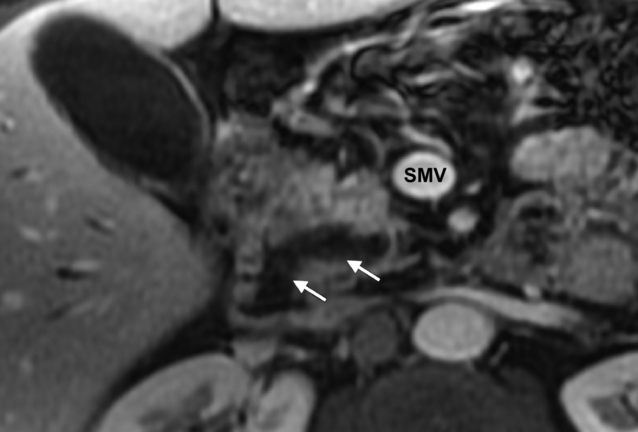
Figure 16.1 MRI (post-gadolinium, T1-weighted, fat-saturated) image of the pancreas. The white arrows indicate a dilated pancreatic duct with a widely open ampulla consistent with a main-duct intraductal papillary neoplasm. SMV, superior mesenteric vein. Histology is shown in Fig. 16.2.
Endoscopic ultrasound (EUS) has the advantage of being able to sample cystic fluid and biopsy solid lesions at the time of assessment, although its utility over cross-sectional imaging has recently been questioned.20 Features seen at EUS suggestive of malignancy include main duct > 10 mm (for main-duct IPMN), while suspicious features for branch-type IPMN include tumour diameter greater than 40 mm associated with thick irregular septa and mural nodules > 10 mm.21 In a series of 74 patients with IPMNs of which 21 (28%) had invasive carcinoma, the sensitivity, specificity and accuracy of EUS fine-needle aspiration in predicting invasive carcinoma were 75%, 91% and 86%, respectively.22 In this particular study, the elevated levels of carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 within cyst fluid did not predict the presence of malignancy.22 Importantly, the absence of mucin does not exclude IPMN.23 While the presence of necrosis is the only feature that is strongly suggestive of invasive carcinoma, abundant background inflammation and parachromatin clearing are suspicious for carcinoma in situ.23
Endoscopic retrograde cholangiopancreatography (ERCP) can be used in the diagnosis of IPMN, although MRI (including the use of gadolinium) is increasingly replacing it (Fig. 16.1). The observation at ERCP of mucin protruding from a widely open papilla is diagnostic.24 Biopsies and aspiration of ductal contents can be obtained; however, the yield is less than 50%.24
Although there are no tumour markers specific to IPMN, serum CA19-9 but not CEA has been shown to be an independent predictor of malignancy.10
Given the increasing frequency of diagnosis and relatively low rate of malignancy within branch-duct IPMN, clinicoradiological scoring systems have been proposed.10,25 Fujino et al. have proposed a clinicoradiological scoring system for predicting the presence of invasive malignancy in patients with both branch- and main-duct IPMNs (based on an analysis of 64 patients who underwent resection).10 It consists of seven factors (Table 16.1), each with an assigned score. A cut-off of 3 or more predicts malignancy with a sensitivity, specificity, positive predictive value, negative predictive value and overall accuracy of 95%, 82%, 91%, 90% and 91%, respectively. No patient with a score of > 4 had benign lesions, while no patient with a score of < 2 had malignancy. Clearly, if this system is validated and further refined with larger numbers of patients, this may prove a very simple and useful predictor of underlying malignancy. In a large study by Hwang et al.,25 237 patients with branch-duct IPMN who underwent resection were studied. Using multivariate analysis to identify independent predictors of either malignancy or invasiveness, formulae were created. However, the presence of a mural nodule, elevated serum CEA or cyst size greater than 28 mm was sufficient to conclude that there was underlying malignant change or invasion and an indication for surgery.25 An important point when considering the use of these scoring systems is that the radiological measurement varies by scan modality and may not correlate well with the final pathological measurement.26
Table 16.1
Proposed scoring system10 to predict malignancy in patients with suspected intraductal papillary mucinous neoplasms of the pancreas
| Variable | Score |
| Patulous papilla | 1 |
| Jaundice | 1 |
| Diabetes mellitus | 1 |
| Tumour size ≥ 42 mm | 1 |
| Main-duct type | 2 |
| Main duct ≥ 6.5 mm | 3 |
| CA 19-9 ≥ 35 units/mL | 3 |
Pathology
IPMNs involve the head of the gland in 70% of cases, while 5–10% are spread diffusely throughout the gland, and the rest are located within the body and tail.27 On sectioning, the involvement can be diffuse or segmented, with projections of papillary epithelium (Fig. 16.2) and tenacious thick mucin within the involved dilated ducts. The projections and mucin can extend along the ducts and into the surrounding structures, including the ampulla, duodenum and bile duct. Communication of the main pancreatic duct with the cystic lesion can usually be established. IPMNs are subclassified into main duct, branch type or mixed, depending on site of origin. This is important as branch-type neoplasms are less likely to be associated with malignancy.24 Surrounding pancreatic parenchyma may appear firm and hard due to scarring and atrophy from obstructive chronic pancreatitis secondary to the tumour. The presence of gelatinous or solid nodules should raise the suspicion of an invasive component. Microscopically, the most typical appearance is of mucin-secreting columnar epithelium with variable atypia (low-, moderate-, high-grade dysplasia or invasive carcinoma).27 The growth pattern varies from flat ducts (ectasia) through to prominent papillae. The tumour tends to follow the pancreatic ducts and can be multifocal in 20–30% of patients.27 IPMNs can contain intestinal, gastric or, less commonly, pancreatico-biliary type differentiation. The gastric type are more often associated with branch-type IPMN and would seem to be associated with a different (lower) malignant potential, growth pattern and type of mucin production compared to the intestinal type.28,29 Invasive carcinoma occurs focally and is thought to result from a stepwise progression through increasingly dysplastic lesions.27 The invasive growth pattern can be muconodular (colloid) or a conventional ductal pattern and would appear to be related to the underlying cellular differentiation (intestinal vs. pancreatico-biliary, respectively).27,29
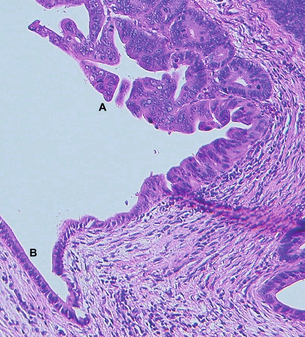
Figure 16.2 Haematoxylin-and-eosin-stained section from the pancreatico-duodenectomy specimen of the patient in Fig. 16.1. Label A is in the lumen of the proximal pancreatic duct with adjacent proliferation of severely dysplastic glandular epithelium with intraluminal papillary growth, but no stromal invasion in this area. Elsewhere in the specimen focal stromal invasion was identified. Label B indicates remnant low columnar non-neoplastic epithelium of the duct.
Pathologically, differentiating IPMN from other cystic neoplasms of the pancreas is important. The absence of ovarian stroma helps to separate IPMN from MCN.4 For lesions between 0.5 and 1 cm, differentiating pancreatic intraepithelial neoplasia (PanIN) from IPMN is difficult. IPMNs tend to have taller and more complex papillae and are associated with abundant luminal mucin.27 The presence of coarse and stippled chromatin with a smooth nuclear membrane will differentiate cystic pancreatic endocrine neoplasms from IPMNs.27
Management
The same IAP guidelines4 recommended that all patients with symptomatic branch-duct IPMNs underwent resection on the basis that it would alleviate symptoms and because the literature would suggest that there is a higher rate of malignancy in patients who are symptomatic (risk of invasive malignancy 30%).4 For asymptomatic patients4 it was recommended that patients with tumours ≥ 30 mm or with mural nodules underwent resection due to the increased risk of malignancy. Although risk factors for malignancy have been identified by more than one study using multivariate analysis,10,30these have been based on small numbers of patients. Nagai et al. have challenged this approach, advocating aggressive surgical resection for branch-type IPMNs, arguing that the identified risk factors do not have a high enough negative predictive value, that survival is significantly compromised in those with invasive disease, and that pancreatic surgery can be performed with a low morbidity and mortality in experienced centres.31
Since publication of the IAP guidelines,4 a large dual-centre study32 consisting of 145 patients with branch-duct IPMNs who underwent resection has been reported. Of these 145 patients, 22% had malignant disease (in situ or invasive) and 40% were asymptomatic. Although symptoms per se were not found to be a predictor of malignancy on univariate analysis, jaundice and abdominal pain were more likely to be associated with malignancy. Radiologically malignant tumours were larger, and on pathological analysis the presence of a thick wall, nodularity and size ≥ 30 mm were all significantly associated with malignancy. It is important to note, however, that other than size these factors were not assessed radiologically. In addition, there was a significant discrepancy between radiologically and pathologically measured size (radiological measurement was consistently 15% greater). The authors concluded that their results supported the IAP guidelines, particularly with regard to non-surgical management of those that were asymptomatic with no concerning features of malignancy.
Given that even branch-duct IPMN would appear to be a premalignant lesion, albeit a slow-growing one, one has to know the outcome from long-term follow-up if conservative management is to be successful. In two large prospective contemporary studies33,34 of branch-duct IPMNs, in which indications for resection were based on IAP guidelines, patients were allocated to a surgical or intensive follow-up arm. In both studies 18% of patients met the criteria for surgery at initial presentation. Of these patients, the final histology was malignant (in situ or invasive disease) in 3 of 2033 and 8 of 3434 patients. In those patients submitted to follow-up, intensive regimens (3–6 monthly for the first 2 years) were used in both studies, including combinations of CT, EUS and MRI. Between 5% and 12% of patients subsequently progressed to surgery during follow-up (median 12–18 months). Of these patients, 0 of 533 and 2 of 1834 had malignant disease. All remaining patients (n = 8433 and n = 13234) that were followed remained alive during median follow-up periods of 30 months, with no deaths attributable to their disease.
For those patients in whom surgery is indicated, the decision regarding the extent of pancreatic resection and nodal dissection needs to be decided. Fujino et al. reviewed the outcome in 57 patients who underwent surgical resection for IPMN.35 Their approach was to perform a localised resection where pre-resection imaging revealed localised disease, using intraoperative ultrasound (IOUS) to determine the point of pancreatic transection, while in patients with diffuse disease a total pancreatectomy was performed. Frozen section was performed and for patients with invasive carcinoma a radical resection was performed. Where non-invasive disease was detected, a tumour-free margin was sufficient. Of the 33 patients with main-duct IPMNs, 14 met the pre-resection criteria for total pancreatectomy. All 24 patients with branch-duct tumours underwent partial resections, although two subsequently required completion pancreatectomy for complications. Correlating the final pathological assessment with the IOUS indicated an accuracy of ductal spread of 74% for main-duct tumours and 96% for branch-duct tumours. Frozen section was performed in 30 of the patients who underwent partial resection and in 29 patients it correlated with the final result. Only one patient had invasive malignancy at the transected surface, while a further two patients who did not have frozen section assessment had invasive malignancy at the resection margin.
Although Fujino et al.35 report frozen section to be very accurate, it can be a challenging undertaking for the pathologist. However, not all positive margins require resection. Current recommendations from the IAP guidelines4 are that, in the presence of adenoma or borderline atypia, no further resection is required, but if in situ or invasive carcinoma is present, then further resection should be performed. However, what has not yet been addressed in the literature is the effect of potentially spilling invasive carcinoma cells (i.e. cutting through invasive tumour) during surgery and the effect this has on long-term outcomes. This is particularly important as increasingly limited resections are being reported for low-grade lesions within the pancreas with good long-term outcomes. However, for main-duct IPMNs, the authors have advised caution for exactly this reason, given the risk of a positive resection margin and subsequent recurrence.36
Outcome
The main determinant of survival following resection is the presence of invasive disease (Table 16.2). The 5-year survival for those with non-invasive disease is 77–100%9,31,33,35,37,38 vs. 13–68%1,9,31,33,35,37–41 for those with invasive disease. Other factors that have been reported to be associated with poor survival in those with invasive disease include the presence of jaundice,42 tumour type (tubular worse than colloid),9,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




