Fig. 7.1
Skin histology. Cryoglobulinemic vasculitis with fibrinoid necrosis of the capillary walls with pyknotic nuclei and nuclear debris of granulocytes and mononuclear cell infiltrates
Rheumatologic Manifestations.
Arthralgias, usually bilateral and symmetric, are present in 40–90 % of patients. The clinical pattern may vary largely among patient series referred to different tertiary care facilities [12, 14, 15]. Arthritis is relatively rare, characterized by two main patterns: oligoarthritis [12] usually mild and nonerosive involving medium-sized and large joints and symmetrical polyarthritis mimicking rheumatoid arthritis. Xerostomia and xerophthalmia are present in 30 % of cases, but very few cases fulfill the classification criteria for Sjögren’s syndrome.
Gastrointestinal Involvement
. Chronic hepatitis is the rule in HCV-infected patients, evolving in cirrhosis in a quarter of patients. The clinical course of chronic hepatitis is generally mild to moderate. Hepatocarcinoma is a less frequent complication than the subjects with HCV-related hepatitis without MC [12]. Episodes of diffuse abdominal pain can be observed in any time of the disease, especially during the flare-up episodes, and range from 7.5 to 11 %. Abdominal pain is an expression of mesenteric vasculitis as confirmed in occasional laparotomy [15].
Neurological Disorders
This is a major cause of clinical disability making the quality of patient’s life severely compromised. Peripheral neuropathy is clinically present in 10–30 % of cases at the onset of the disease and increases in the follow-up. If peripheral neuropathy is searched by mean of electrophysiology techniques, it can be detected in the vast majority of the patients. The common symptoms are paresthesias with painful or burning sensations of the lower limb with nocturnal exacerbation. Peripheral neuropathy is sensory or sensory motor and mainly asymmetric, more often presenting as a mononeuritis multiplex. Axonal damage is thought to be related to cryoglobulin occlusion or perivascular inflammation of vasa nervorum. Anti-neuronal antibodies could play an additional role [44]. Seizures and dysarthria have been reported on occasion [15]. Exceptionally, cerebral hemorrhage, as an expression of brain vasculitis, can occur [13, 15].
Cardiovascular Manifestations.
Myocardial infarction, angina, and myocardial insufficiency are generally absent at the onset of the disease but became progressively more frequent in the follow-up as a consequence of a high prevalence of hypertension. Coronary vasculitis, congestive heart failure, and episodes of pericarditis can occur in the course of the disease [15].
A hyperviscosity syndrome related to high levels of cryoglobulins, which represents a major symptom of a monoclonal cryoglobulinemic syndrome related to a hematologic disorder, is rare in mixed cryoglobulinemia [12].
Hypertension
The prevalence of hypertension at the time of renal biopsy is about 75 %. During the follow-up, the arterial pressure is hardly controlled. More than 50 % of the patients involved in a multicenter trial who were followed up for at least 10 years showed pathologic values [15].
Bone Marrow Abnormalities
This is the most frequent neoplastic complication and is related to the lymphoid infiltrates, in the form of “early lymphomas,” found in liver, spleen, and bone marrow. These infiltrates tend to remain unchanged even for decades. The term “monotypic lymphoproliferative disorder of undetermined significance” has been coined. In about 10 % of cases, an overt lymphoma can develop.
Lung Involvement
It is usually asymptomatic, but diffuse interstitial pulmonary fibrosis can be observed on occasion. Alveolar hemorrhage is a rare event.
Laboratory Markers
Biochemical analyses usually reveal type II cryoglobulins (IgM–k, polyclonal IgG), IgM RF ranging from slight to extreme increase, and low values of C3, C4, and C1q. C4 levels are usually very low, sometimes undetectable, and represent a valid surrogate marker of cryoglobulinemia. Anemia is the rule, due to chronic inflammation and, when present, renal failure. C-reactive protein and erythrocyte sedimentation rate are usually high [15]. Inexplicable fluctuations of erythrocyte sedimentation rate and/or immunoglobulin levels sometimes reflect untimely processing of the blood specimen. A sudden disappearance of cryoglobulin and rheumatoid factor or a spontaneous increase of C4 can announce the development of an overt lymphoma.
A critical issue is cryoglobulin detection. A negative cryoglobulin detection does not a priori rule out the diagnosis, since cryoglobulins precipitate when the test sample cools below 37 °C during transit to bedside to laboratory, leading to false-negative results.
A recommended method for detection of cryoglobulins is as follows. Venous blood is collected without anticoagulation in a pre-warmed syringe, transferred in warmed tubes, and stored at 37 °C until it clots. After centrifugation at 37 °C, the serum is collected and stored at 4 °C for 1 week. After separation and washing, the cryoprecipitate is quantified and expressed as percentage of precipitate/serum volume. The components of cryoprecipitate are determined by immunoelectrophoresis or immunofixation. Processing of anticoagulated blood can produce false-positive results due to cryofibrinogen or heparin-precipitable proteins. If the disease is highly suspected and the cryoglobulins cannot be detected by this method, a simple technique of precipitation in hypoionic medium to detect the so-called hypocryoglobulins can be used [45]. Could you give a reference for this? Please refer to n° [15]. Serum collected after centrifugation is mixed volume to volume with be-distilled water before storage [15].
The levels of serum cryoglobulins do not correlate with the severity of the disease. Low levels of cryocrit, even in trace amounts, such as those found using the method of hypocryoglobulin detection, can be associated with severe cryoglobulinemic syndrome. Conversely, high cryocrit values can be related to a mild clinical course.
With sensitive methodologies, such as immunoblotting or two-dimensional polyacrylamide gel electrophoresis, oligoclonal IgM with polyclonal IgG can be detected. This particular pattern represents an intermediate, possibly evolutive state from types III to II mixed cryoglobulin and is called “types II–III cryoglobulin.”
Renal Histology
Light Microscopy
Despite the variety of clinical presentations, three main glomerular patterns can be recognized [15].
Diffuse Membranoproliferative GN (About 80 % of Cases)
. This is the typical pattern of cryoglobulinemic glomerulonephritis (Figs. 7.2 and 7.3) characterized by duplication of glomerular basement membrane, interposition by mesangial cells, and especially mononuclear leukocytes/macrophages, subendothelial (but also mesangial) deposition of immune reactants, mesangial expansion and proliferation with intracapillary leukocyte accumulation, and endoluminal hyaline thrombi (corresponding to cryoglobulin precipitates). More than 50 % of glomeruli are involved. Immune deposits are eosinophilic and PAS positive, red/orange by trichrome stain. A centrolobular sclerosis can be detected in half of cases. Extracapillary proliferation is relatively uncommon and usually affects a minority of glomeruli. Exceptionally, crescents involve more than 50 % of glomeruli. Necrosis of the glomerular tuft can be found on occasion.
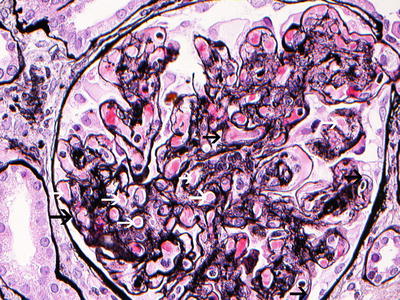
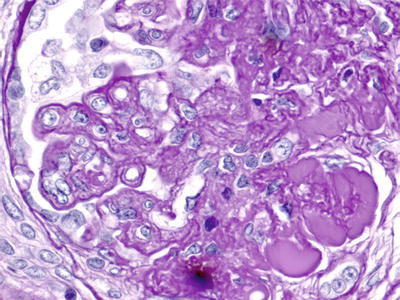

Fig. 7.2
Light microscopy. Silver stain (×40) showing a membranoproliferative pattern of injury with double contour formation (black arrows). Many loops contain pale eosinophilic material consistent with cryoglobulins (white arrows)

Fig. 7.3
Light microscopy ×1,000. PAS + ve endoluminal pseudothrombi and reduplication with cellular interposition of the glomerular basement membranes
Focal Membranoproliferative Glomerulonephritis (About 10 % of Cases).
The pattern of cryoglobulinemic glomerulonephritis is typical but involves less than 50 % of glomeruli. Cellular proliferation and exudation and thickening of the capillary wall are mild, with irregular distribution within the same glomerulus and among glomeruli. Endoluminal thrombi are less frequently found than in the diffuse form.
Mesangial Proliferative Glomerulonephritis (10 % of Cases).
It is characterized by diffuse mesangial expansion and proliferation without exudation and endocapillary proliferation. Rarely isolated proteic endoluminal thrombi can be detected.
Interstitial and Vascular Lesions
Interstitial leukocyte infiltration, usually focal, is present more frequently in the membranoproliferative forms and is correlated with the intensity of the proliferative glomerular lesions. Interstitial fibrosis, mainly focal, is almost invariably present in the membranoproliferative forms. Arteriosclerotic lesions are present in one third of cases with no differences among groups. Arteritis is relatively rare.
Immunofluorescence Microscopy
Diffuse, pseudolinear peripheral capillary wall and mesangial staining for IgM, IgG, and C3 are usually found, with a relatively stronger staining for IgM and k (as compared to lambda) light chain, which reflects the typical clonal restriction of type II cryoglobulins (Fig. 7.4). Prominent IgM and IgG staining is detected in thrombi. Fibrinogen is found in vessel walls when a vasculitis is present.
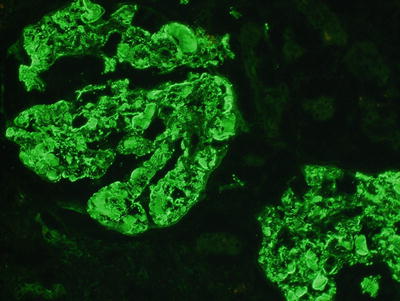

Fig. 7.4
Immunofluorescence studies showing cryoglobulins in many capillary loops that are positive for IgM
Electron Microscopy
Electron-dense deposits are detected in subendothelial and mesangial areas. Segmental subepithelial deposits are occasionally seen. Deposits can be also detected within phagolysosomes of intracapillary macrophages. Interposition of glomerular basement membrane by monocytes is seen (Fig. 7.5). As discussed above, the role of these cells in cryoglobulin removal is a controversial issue. Cryoglobulin deposits often display an organized substructure: short, curved, thick-walled tubular structures with a diameter of about 30 nm which appear annular on cross sections (Fig. 7.6).
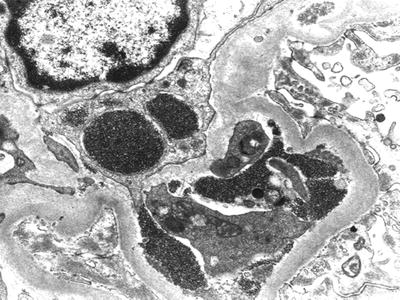
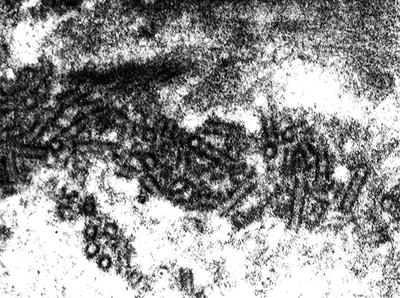

Fig. 7.5
Electron microscopy. Monocyte interposition into the glomerular basement membrane. Monocytes are engulfed with cryoglobulins

Fig. 7.6
Electron microscopy. Magnified pictures of the characteristic tubular and anular structures (about 30 nm of diameter) of mixed cryoglobulins
Atypical Features
A few cases of membranoproliferative glomerulonephritis present, in addition to the common immunofluorescence pattern, deposits of IgA with prevalent mesangial but also parietal localization [15]. In a small percentage of patients, a pattern of membranous nephropathy can be detected. It is characterized by the same morphology of the primary form, with prevalent subepithelial deposits of IgM, IgG, and C3 (with crystalloid structured deposits at the electron microscopy examination). Segmental features of membranoproliferative glomerulonephritis together with membranous nephropathy can be detected in rare cases.
Clinical-Pathologic Correlations
The patients with diffuse membranoproliferative glomerulonephritis show a stronger C4 hypocomplementemia and higher levels of proteinuria than the patients with other patterns [4]. Hematuria and hypertension are equally distributed among the various histologic groups. Serositis, hepatosplenomegaly, leukopenia, peripheral neuropathy, and cardiac involvement are more frequently observed in association with the diffuse membranoproliferative pattern [15].
Prognostic Factors
Significant prognostic variables include age, male gender, creatinine and proteinuria at the time of renal biopsy, number of syndromic relapses, and poor blood pressure control [15]. Survival at 10 years is about 80 % nowadays [15], much better than a decade ago [13]. Kaplan–Meier survival curves are worsened by basal creatinine value >1.5 mg/dL (133 μ[mu]mol/L). Cardiovascular disease is the cause of death in over 60 % of cases.
Non-Cryoglobulinemic HCV-Associated Glomerulonephritis
Due to the diverse histologic patterns and the specific therapeutic implications (see below), biopsy should be done, if not specifically contraindicated, in every patient with mixed cryoglobulinemia who presents with urinary abnormalities or otherwise unexplained renal insufficiency.
A number of alternative renal manifestations have been reported in hepatitis C-infected patients, including membranous nephropathy [46], focal segmental sclerosis [47], IgA nephropathy and other proliferative glomerulonephritis [48], non-cryoglobulinemic membranoproliferative glomerulonephritis [49], fibrillary and immunotactoid glomerulopathies, and anti-cardiolipin-associated thrombotic micro-angiopathy [50].
Treatment
Antiviral Therapy
Discovery of an association between mixed cryoglobulinemia and HCV and the possible pathogenetic implications prompted researchers to develop new approaches to disease control by eradicating the infection. Interestingly, due to its antiproliferative and immunomodulatory effects, interferon-α[alpha] (IFN) was used, and it achieved favorable results in the treatment of mixed cryoglobulinemia even before HCV had been identified [51].
Antiviral treatment of mixed cryoglobulinemia followed the evolution of treatment of chronic hepatitis C. However, studies on antiviral therapy of mixed cryoglobulinemia are more difficult to compare because of a greater heterogeneity in treatment regimens, patient selection, response evaluation, and short follow-up [52–60]. The presence of circulating cryoglobulins should not influence the response to IFN in patients with chronic HCV infection [53–55], albeit cryoglobulinemia probably favors HCV entrapment inside mega complexes which are phagocytosed, but not degraded by infected phagocytes [41] that are potential virus reservoirs. Actually, in a very large cohort of nephritic patients, less than 10 % of subjects reached sustained viral response (SVR), i.e., undetectable HCV-RNA 24 weeks after the end of treatment [56], using standard interferon [15].
With regard to the effects of IFN on syndromic symptoms, a number of retrospective studies have reported some clinical improvement in about 60 % of patients with mixed cryoglobulinemia [57–62]. In responsive patients, reduction of HCV-RNA heralded the decline of cryocrit, IgM, and RF activity. Notably, in Dammacco’s study [59], the concomitant administration of prednisone resulted in earlier responses of cryoglobulinemic symptoms than with interferon-α[alpha] alone.
The attempts to identify clinical or serological pre-therapy variables predictive of interferon-α[alpha] response are controversial. In Mazzaro’s study [62], the lack of response was associated with genotype 1b, liver cirrhosis, and high cryoglobulin levels. The HCV-RNA levels might also affect response. Casato et al. [61] found a correlation between detection of anti-HCV core antibodies and good response. Purpuric lesions tend to respond rapidly while neuropathy and nephropathy are the slowest to respond.
In these studies, the benefit of IFN monotherapy was transient, and relapse was very frequent after therapy discontinuation [53, 57–72] and often combined with the reappearance of viral RNA, occasional worsening of skin ulcers (possibly due to the antiangiogenic effects of alpha interferon), precipitation of renal failure and nephrotic syndrome, psychiatric manifestations, peripheral neuropathy, and autoimmune hepatitis.
Trials with IFN plus ribavirin (RBV) showed better performance in both viral eradication and cure of symptoms [73–78]. Ribavirin, an oral guanosine nucleoside analog that induces a significant decrease in serum aminotransferase, is unable to achieve a long-term virological and biochemical response when given alone. It has rarely been used as monotherapy in patients with HCV-associated glomerulopathy, and it has achieved limited response in both immunocompetent and transplanted patients.
At present, ribavirin is almost only used in combination with interferon-α[alpha] [73–75, 77], but only few studies have been reported in the literature. Calleja et al. [74] treated 13 patients with interferon-α[alpha] and ribavirin for 12 months. All patients had previously received interferon-α[alpha] in monotherapy. Five of the eight nonresponders to interferon alone showed an initial response, but this was sustained in only three cases after 12 months. Loss of response was accompanied by the reappearance of HCV-RNA, worsening of clinical manifestations and an increase in the levels of cryoglobulins and hepatic enzymes.
All nine patients who were refractory to interferon-α[alpha] treated by Zuckerman et al. [75] with interferon-α[alpha] and ribavirin for 6 months achieved a substantial improvement in mixed cryoglobulinemia-related symptoms although polyneuropathy was resistant to treatment.
Sustained response has also been reported by Donada et al. [73]. In Mazzaro’s report [77], 18 % of patients who were “relapsers” to the first interferon-alpha treatment had complete recovery from viral infection and from all signs and symptoms of the disease after 1 year of combined treatment. A marked clinical improvement occurred in the majority of cases within 2 or 3 weeks of treatment. Regrettably, 70 % of patients relapsed within a few weeks of the end of treatment.
Finally, a single study showed an improvement in renal histology in a few patients with biopsy-proven GN who underwent antiviral combination therapy with alpha interferon and ribavirin [78].
Care should be taken when administering ribavirin in patients with severe renal impairment as this drug is not dialyzable. Mild dose-related hemolytic anemia is commonly observed in patients treated with the combination therapy.
Antiviral treatment with IFN-α[alpha] also proved to be effective in determining some regression of monoclonal B-cell expansion. Several series reported that antiviral treatment with interferon-α[alpha] alone or with ribavirin is effective in HCV-associated indolent and marginal zone lymphoma accompanied with cryoglobulinemia [79–82].
Further progress with regard to response to therapy has been obtained using pegylated interferon (PEG-IFN) which is endowed with improved pharmacokinetic characteristics. PEG-IFN alone or combined with ribavirin has proven to be more effective than interferon-α[alpha] alone, or plus ribavirin, in patients with HCV infection, especially those infected with genotype 1b. Currently, PEG-IFN in combination with ribavirin is the standard treatment for chronic hepatitis C, with 41–48 % SVR in genotype 1 and approximately 80 % SVR in genotypes 2 and 3.
This combined therapy also opened new opportunities for the treatment of mixed cryoglobulinemia and is presently regarded as the first-line antiviral treatment for these patients.
Indeed, patients with HCV-related mixed cryoglobulinemia seemed to benefit from this new combination therapy much more than from standard therapy, even though, as in Mazzaro’s study [83], 44 % of patients relapsed a few weeks after the end of therapy. However, Cacoub et al. [84] obtained remarkable results in clinical, virological, and serological parameters in 9 patients with cryoglobulinemic vasculitis.
Nowadays, the therapeutic strategy for HCV eradication in chronic hepatitis C with the combination of PEG-interferon and ribavirin is based on genotype. Genotypes 2 and 3 can be eradicated in 75–80 % of patients (obtaining sustained virological response) by a 6-month combined regimen. Conversely, genotype 1b requires a 48-week course of therapy with weekly injections of either PEG-IFN-α[alpha]2a or α[alpha]-2b and 1,200 mg of oral ribavirin daily. In this case, the rate of sustained virological response is only 41–48 %, and in the absence of a decrease in HCV-RNA concentration of 2 logs at week 12, the chances of responding is further reduced (up to 20 %). This figure is even lower in African Americans and individuals with advanced fibrosis or HIV coinfection. Therefore, a considerable percentage of patients with HCV-associated mixed cryoglobulinemia are expected not to achieve virological response and to have persistent cryoglobulins and symptoms [85, 86]. Moreover, as emphasized above, due to interferon immunomodulatory properties, antiviral treatment can induce immune-mediated adverse events, such as peripheral sensory-motor neuropathy, thyroiditis, rheumatoid-like polyarthritis, and other vasculitic manifestations [57, 87–92] that may precipitate or exacerbate preexisting, subclinical disorders. Unfortunately, there are no available parameters for predicting these complications. Thus, antiviral therapy should be very carefully administered to patients with mixed cryoglobulinemia-related peripheral neuropathy, active skin ulcers, or severe nephritis. In addition, several patients may present clinical conditions, including advanced age, decompensated cirrhosis, major uncontrolled depressive illness, significant coronary heart disease, and untreated thyroid disease [56], which make antiviral treatment not recommended.
In conclusion, acute nephritic syndrome, extensive cutaneous ulcers, widespread vasculitis, and hyperviscosity syndrome actually do require more prompt and aggressive treatment, even due to the uncertain response to antiviral therapy. In these settings, antiviral therapy may only have a role in sequential or combined treatment schedules [52, 93–95].
In clinical practice, antiviral treatment of mixed cryoglobulinemia should be tailored to the individual patient according to the progression and severity of clinical manifestations [93, 94].
Randomized controlled trials of adequate size and follow-up are needed to clarify whether higher virological or clinical response rates can be obtained by extending treatment duration (up to 48 weeks for HCV genotypes 2 or 3 and 72 weeks for HCV genotypes 1 or 4).
Lastly, the role of Telaprevir, the promising peptidomimetic inhibitor of NS3–4A protease, that is able to increase the rate of SVR in patients with genotype 1 from 41–48 % to 61–68 % when given in combination with standard INF/Ribavirin regimen [96], has not yet been explored in cryoglobulinemic patients.
Standard Immunosuppression
Despite the unquestionable evidence of a viral etiology, immunosuppression is still regarded as the first-line intervention if renal involvement is severe. Immunosuppression may also be taken into consideration in patients failing to respond to interferon treatment or in acute immunological flares. In these cases antiviral treatment is usually either insufficient to control renal disease or even detrimental. In many centers, high-dose corticosteroids, plasmapheresis, and, in more severe cases, cytotoxic therapy are commonly administered, but no trials have prospectively evaluated these treatments. These therapeutic approaches may cause an increase in the levels of viremia, thus exacerbating chronic hepatitis C disease. Nevertheless, renal disease is often a compelling indication for these treatments. Purpura is the most frequent indication for corticosteroids, which remain the most amenable to therapy. Another indication is peripheral neuropathy, even though its general refractoriness to any treatment is widely recognized. Patients who need such therapies usually require multiple courses. Symptoms often recur after periods of quiescence or flare after periods of stability.
By examining the data of a robust sample of patients with cryoglobulinemic nephritis [15] and comparing them to a similar cohort described 10 years earlier [14], a better survival rate (80 % vs 50 % at 10 years) was shown with cardiovascular events as predominant cause of death instead of infections and hepatic failure observed in the previous study. There could be several reasons for this changing feature. The better survival profiles which have been obtained over the last decade could be the consequence of aggressive but time-limited intervention on the acute renal involvement, together with the long-term antiviral therapy that was used to control moderate manifestations of renal and extrarenal involvement.
More specifically, with regard to glucocorticoids, long-term administration of low-medium doses (0.1–0.5 mg/kg/day) of steroids alone was largely used in clinical practice for vasculitis symptoms or pain, and data from small case series support their effectiveness in controlling the flares of disease [59, 97–99]. However, the result of controlled studies have been conflicting [59, 99–101], and no reports evaluating the effectiveness of long-term glucocorticoid administration are available, while side effects of long-lasting steroid therapy are known to be serious and irreversible. Glucocorticoids, also in pulse doses, are frequently used in association with other drugs [59, 97–103]. As far as cytotoxic drugs are concerned, while there is a lack of significant experience with the use of cyclosporine, azathioprine, methotrexate, and mycophenolate mofetil, and what there is remains anecdotal, cyclophosphamide was frequently used in mixed cryoglobulinemic patients [104–112], especially in association with apheresis. The rationale of cyclophosphamide administration in this setting is based on the assumed need to obtain temporary immunosuppression after acute apheretic treatment, but neither randomized controlled data nor large cohort studies support this postulate. Cyclophosphamide has been taken into consideration mainly for the treatment of membranoproliferative glomerulonephritis or severe polyneuropathy. The majority of data was collected before HCV was identified as a major determinant for mixed cryoglobulinemia, and the risks of using cyclophosphamide in patients with HCV infection are poorly defined. The use of cyclophosphamide in an i.v. bolus is currently believed to be safer than oral administration, reducing the cumulative dose [113]. Careful monitoring of HCV infection and liver function after cyclophosphamide administration is recommended.
With regard to plasma exchange, several different apheretic procedures have been applied in the treatment of various clinical conditions associated with mixed cryoglobulinemia. No controlled trials or large cohort studies are available on this issue. Data can only be drawn from case reports [97, 104–120], which were often published before the association between HCV and mixed cryoglobulinemia was discovered, and furthermore, the apheresis methods significantly differ in the various studies.
Several observations support the role of apheresis both in improving acute renal disease [115–117] and in treating neuritis [102, 115] and ulcers [97, 120]. With regard to hyperviscosity syndrome, despite the lack of data from controlled randomized studies, which are obviously difficult to obtain in this rare condition, apheresis procedures remain the treatment of choice. A combination of immunosuppressant drugs in association with apheresis has been supported by some clinical reports [107–112], but is no longer considered mandatory.
Among the available apheretic approaches, double filtration (which selectively removes molecules greater than 600 kDa) is the procedure of choice [120]. No agreement exists about intensity, duration, and frequency.
In clinical practice, Cyclophosphamide (1.5–2 mg/kg/day given orally for 3 months, or 0.5–1 g administered intravenously every 2–4 weeks), in association with oral steroids (0.5–1 mg/kg/day for 1 month with subsequent tapering by 2.5–5 mg/week) often preceded by 3 pulses of 10–15 mg/kg methylprednisolone, represents the most widespread combination therapy in the severe manifestations of mixed cryoglobulinemia or in patients with uncontrolled symptoms.
The less toxic mycophenolate mofetil given for 6 months can be an alternative option.
It has recently been claimed that Cyclosporin is able both to interfere with the polymerase binding affinity to viral RNA [121] and to achieve a significant decrease in viral load after a few months of administration in patients with HCV-associated non-cryoglobulinemic arthritis.
Plasma exchange, especially double-filtration plasmapheresis, continues to be successfully used in escalation protocols for rapidly progressive glomerulonephritis, skin ulcers, sensory-motor neuropathy, widespread vasculitis, and hyperviscosity syndrome. A recommended scheme consists of a procedure every other day for 2 weeks followed by two procedures a week for 2 weeks and one procedure a week for a month [113].
A standard course of combined antiviral therapy is indicated following immunosuppression in order to attenuate viral replication.
Novel Therapeutic Strategies
A critical aspect in the development of novel therapeutic strategies is the validation of treatment options that are alternative to steroids given alone or in combination with immunosuppressive drugs in the acute manifestations of cryoglobulinemia. Such alternatives could further prolong patient survival (which has already been improved in the last decade [15]) by reducing cardiovascular risks.
Among the biologic drugs currently used in immunopathology that have raised hopes for new therapeutic approaches for patients with systemic signs of severe vasculitis, Rituximab (RTX) and Infliximab are the only two that have been employed in the treatment of mixed cryoglobulinemia. Encouraging results have been obtained with Rituximab in open studies and single case reports [122–129]. Initial results with Infliximab [130–132] did not support the extensive use of this drug in mixed cryoglobulinemia.
Rituximab is a humanized mouse monoclonal antibody directed at CD20, a B-cell-specific membrane protein. Presently, the published cases of mixed cryoglobulinemia treated with Rituximab account for more than 300 patients, deriving from 20 case reports, 13 clinical studies with at least five patients [122–124, 126, 129, 133–140], three trials in which Rituximab was associated with PEG-IFN plus Ribavirin [95, 139, 141], one retrospective study on the effects of the combination of Rituximab with antiblastic therapy [142], and, finally, three studies [135, 143, 144], two randomized [143, 144], that will be considered separately. General data have been collected in [140].
The lymphoma protocol, consisting of 4 weekly infusions of 375 mg/m2, was usually used. Two more doses at 1-month intervals were administered in two studies [124, 129]. No additional therapy was given in 19 nephritis patients [124, 126, 129], suggesting that some severe cases could be treated ab initio without steroids.
Rituximab was shown to improve or cure various clinical manifestations of mixed cryoglobulinemia, including fatigue and arthritis (in almost all patients), skin manifestations (purpura, skin ulcers), and glomerulonephritis in 75–90 % of cases, peripheral neuropathy in 70 %, abdominal vasculitis in 80 %, and hyperviscosity syndrome. Rituximab was also reported to be effective in some life-threatening conditions.
Glomerulonephritis and skin ulcers usually improve within 3 months after the beginning of therapy. Complete healing takes longer. One study showed that both sensitive and motor neuropathy improved 1–5 months after RTX, and the electromyography picture was stable or had improved [136]. Moreover, RTX treatment reportedly decreases serum cryoglobulins and rheumatoid factor and increases C4 levels, with the disappearance of bone marrow B-cell clonal expansion [95, 124, 129, 145, 146].
Short-term infusion reactions following RTX administration do not appear to be more frequent in mixed cryoglobulinemia than in other immunological-mediated disorders including rheumatoid arthritis. The risk of serum sickness [147] is negligible. Severe adverse effects (1 %) and disease worsening (2 %) are relatively rare [140]. Dropout due to adverse events is less than 5 %. One French group reported that patients with high cryocrit may experience severe flares of vasculitis within 2 days of RTX infusion, especially if the rheumatoid arthritis scheme (two infusions of 1 g/day 2 weeks apart) is used [148]. Plasma exchange before RTX administration has been suggested in patients with high cryocrit levels [148]. However, in clinical practice, slow administration of the lymphoma dose (375 mg/m2) over 12–24 h or administration of half a dose per day in two consecutive days given together with a premedication with steroids, antihistamine drugs, and paracetamol reduces the risk of such reactions [124, 129, 140]. Similar precautions should be taken for patients with a history of heart failure or arrhythmia. Aspirin administration has been proposed for cardiovascular risk [122, 124]. RTX treatment does not significantly affect HCV viral load nor parameters of liver impairment. Presently, there are no data supporting a substantial risk of liver toxicity directly caused by RTX or due to HCV reactivation, even in the long term [129]. Moreover, RTX was successfully prescribed to mixed cryoglobulinemia patients with liver cirrhosis. Patients showed improvement of cryoglobulinemia-related symptoms as well as liver function despite a transient increase in serum HCV-RNA [137, 149]. By contrast, RTX is definitely contraindicated in HBsAg-positive patients because of the possible severe reactivation of HBV infection. In HBsAg-negative/anti-HBc-positive patients (potential carriers [150]), RTX should be used in association with antiviral therapy even if HBV DNA is negative.
Severe infections (lethal disseminated cryptococcosis and severe bacterial pneumonia) were reported after RTX administration in severely immunocompromised renal transplant recipients with type III cryoglobulin-related graft dysfunction [128, 133]. However, except for transplanted patients, no life-threatening infections have been reported in typical HCV-related mixed cryoglobulinemia after RTX administration.
Special care should be taken for the prevention and management of infections, especially in patients who have previously been treated with immunosuppressants or steroids, or those with low serum levels of immunoglobulins. On the contrary de novo hypogammaglobulinemia has rarely been described after RTX in mixed cryoglobulinemia or in other autoimmune diseases [151]. Panniculitis [122], neutropenia [95, 122, 126, 152], and retinal vascular occlusion [122] have seldom been reported as side effects of RTX treatment in mixed cryoglobulinemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







