Crohn’s Disease and Indeterminate Colitis
Victor W. Fazio
The knife cannot always have fresh fields for conquest; and although methods of practice may be modified and varied, and even improved to some extent, it must be within a certain limit.
–SIR JOHN ERICHSEN, Lancet. 1873;2:489
▶ CROHN’S DISEASE
Crohn’s disease of the bowel was initially described by Crohn, Ginzburg, and Oppenheimer in 1932, at which time they noted a transmural inflammatory condition of the terminal ileum.96 The authors apparently listed their names in alphabetical order for the purpose of publication. It would certainly appear that if one is concerned about eponymous immortality, it is helpful to have a name occurring early in the alphabet. Interestingly, many of the cases were based on the large patient experience of Berg.340 Had Dr. Berg wished to include his name on the paper, the condition today would probably be termed Berg’s disease. Ginzburg reflected on the myths and misunderstandings concerning the evolution of the concept of Crohn’s disease in his interesting paper “The Road to Regional Enteritis.”180 A number of publications followed from the experience of Crohn and his associates, confirming the location in the small bowel, but also noting that in a number of cases the colon was to some extent involved.
Another interesting historical footnote was suggested by Goligher189 that Crohn’s disease was actually initially described in 1907 by Lord Moynihan when Moynihan presented his experience with six patients who harbored benign lesions that mimicked carcinoma.368 In 1913, Dalziel reported an obscure tuberculosis-like condition that he called “chronic interstitial enteritis” but which must have been Crohn’s disease.99 In 1923, Moschowitz and Wilensky described four patients with a granulomatous disease of the intestine and the amelioration of the condition by intestinal bypass.366
In 1951, Marshak noted the radiologic findings of what he felt was granulomatous disease of the colon, a clinical entity distinct from that of ulcerative colitis.340 This view was not generally accepted until 1959, when Morson and Lockhart-Mummery described the characteristic pathologic features of granulomatous colitis.364 It can be appreciated, therefore, that our concepts of disease involvement in this area are scarcely one-half century old.
Incidence, Epidemiology, Etiology, and Pathogenesis
The incidence, epidemiology, and theories concerning the etiology and pathogenesis of both Crohn’s disease and ulcerative colitis are discussed in Chapter 29. Inflammatory bowel disease (IBD) remains a complex polygenic disorder interacting with environmental factors that trigger abnormal responses in genetically susceptible individuals.394 The incidence of IBD development in identical twins is 60% in Crohn’s but only 15% in ulcerative colitis.483 Subclinical intestinal inflammation has also been documented in symptomatic first-degree relatives of Crohn’s disease patients.
It is interesting to note that Mycobacterium paratuberculosis DNA has been found in Crohn’s diseased tissue.482 The concept of a bacterial cause for IBD has been discussed in Chapter 29, an issue that has generally been refuted. In one study, however, M. paratuberculosis was identified in 65% of specimens in individuals with Crohn’s disease but in only 4.3% of those with ulcerative colitis.482 The control tissues were found to have this DNA element in 12.5%. It was concluded that these observations are consistent with an etiologic role for M. paratuberculosis in Crohn’s disease. A statistically significant association between the onset of Crohn’s disease and prior antibiotic use has been demonstrated.75 This suggests that a change in the bacterial environment within the intestinal tract in a susceptible host may be responsible for triggering the disease in some patients. Smokers are also overrepresented in Crohn’s patients and underrepresented in ulcerative colitis, and they have an increased risk of recurrence after surgery when compared with nonsmokers.598,603
BURRILL BERNARD CROHN (1884-1983)
 |
Burrill Crohn was born in New York City, June 13, 1884. He graduated from the City College of New York in 1902 and received his medical degree from Columbia University College of Physicians and Surgeons in 1907. Crohn began his internship at Mount Sinai Hospital, the institution with which he was affiliated for his entire professional life. In 1920, he was named the first head of the department of gastroenterology. Henry Janowitz wrote that the “eponym [of Crohn’s disease] is deserved not because of a fortuitous alphabetical listing, but because for many years Crohn alone called attention to this enigmatic inflammation of the bowel, by carefully collecting cases and by publishing his clinical observations.” Janowitz further stated that although not displeased with the honor and the name recognition, Crohn was always modest with respect to his role in the original description. Crohn himself expressed the feeling that the name Crohn’s disease was inappropriate despite its virtually universal use, preferring instead the term regional enteritis. In 1956, when President Dwight D. Eisenhower required surgery for ileitis, it was Crohn who was called to the White House to act as spokesman to explain the disease and the prognosis to the American people. Crohn authored three texts and more than 100 scientific papers. Among his numerous awards were the Townsend Harris Medal by the City College of New York, the Julius Friedenthal Medal of the American Gastroenterological Association, and the Mount Sinai Hospital’s Jacobi Medal. He was also elected president of the American Gastroenterological Association. Crohn died in New Milford, Connecticut, at the age of 99.
LEON GINZBURG (1899-1988)
 |
Leon Ginzburg was born in Bayonne, New Jersey. He completed his undergraduate studies at Columbia University in 1920 and went on to accomplish his surgical training at the Mount Sinai Hospital. Following a tour of the major European institutions, he returned to become A. A. Berg’s House Surgeon at Mount Sinai. For the ensuing 5 years, he was an adjunct on Berg’s ward service and his assistant in the private practice of surgery. The association with Mount Sinai lasted for 40 years, with Ginzburg achieving the rank of clinical professor. From 1947 to 1967, he was director of surgery at the Beth Israel Hospital, as the medical center was then known. The recognition of ileitis was accomplished by examining surgically excised specimens, which led in 1927 to his first description. There was a well-recognized controversy between Ginzburg and Crohn concerning each individual’s respective role in the early observations. As both physicians neared their 90s, Ginzburg compared the discovery of regional ileitis to the controversy over the naming of the United States after Amerigo Vespucci rather than after Christopher Columbus. He likened the former map maker to Crohn, who spent considerable time and effort traveling, lecturing, and spreading knowledge about the disease, while he credited himself with the original description. Leon Ginzburg was active in practice at Beth Israel Medical Center until his death in 1988 at the age of 89. (With special appreciation to Lester Rosen, MD.)
GORDON DAVID OPPENHEIMER (1900-1974)
 |
Gordon Oppenheimer was born on June 30, 1900, in New York City and received his baccalaureate from Columbia College in 1919, and his medical degree from Columbia College of Physicians and Surgeons in 1922. He then became a house officer at the Mount Sinai Hospital, eventually entering the pathology laboratory where he collaborated with Ginzburg in his work on the study of inflammatory lesions of the terminal ileum. Oppenheimer ultimately became a urologist, rising to the position of Chief of Urology at Mount Sinai Hospital, a post he occupied from 1947 to 1963. He authored 69 papers, including a monograph he published with Leon Ginzburg, Urological Complications of Regional Ileitis. In addition to his responsibilities as chairman of the department and director of the residency program, Oppenheimer found time to serve for 14 years as a medical officer with the New York City Fire Department. During World War II, he acted as Second in Command of the General Surgical Service at Mount Sinai Hospital. He is remembered as a kind, gentle, and humble man, an especially humane physician who was much sought after as a consultant urologist. He died of cardiac failure, December 9, 1974, at the age of 74.
ALBERT ASHTON BERG (1872-1950)
 |
It might seem incongruous that I have elected to include A. A. Berg as an individual to be recognized with a biographic sketch in this text. But he represents for me a special person—someone who could afford the “luxury of integrity.” Berg declined to add his name to the alphabetical listing of coauthors because, even though the publication was based on his surgical patients, he did not contribute to the writing. He was born in New York City, August 10, 1872, and attended City College of New York, graduating from the College of Physicians and Surgeons at Columbia University in 1894. After his surgical training at the Mount Sinai Hospital, he joined the staff. In his early years, he was an assistant to Arpad Gerster, the man who introduced Listerian principles to the United States. Berg developed an enormous clinical practice, arguably the largest in the city of New York, having been recognized as a phenomenal technician. He is credited with having performed the first gastrectomy for ulcer disease in the United States. In 1930, he published his experiences with more than 500 patients on the morbidity and mortality of subtotal gastrectomy in the management of gastric and duodenal ulcer. In 1905, he published a text, Surgical Diagnosis: A Manual for Students and Practitioners. In 1934, when he retired from the teaching service at Mount Sinai, the hospital published The Surgical Technique of Dr. A.A. Berg: A Tribute to Forty Years’ Service at the Mt. Sinai Hospital. The chapters were written by his students, including contributions on the small bowel and colon by Leon Ginzburg. Along with his brother (an internist), he amassed a library of 50,000 rare volumes of English and American literature, bequeathing the collection to New York University, Mount Sinai Hospital, and the New York Public Library. Today there exists at Mount Sinai a Berg Laboratory Building and an Institute for Research, and at the New York Public Library, a Berg Room, where the collection is housed. Albert Berg died following kidney surgery on July 1, 1959, at the age of 77.
BERKELEY GEORGE ANDREW MOYNIHAN (1865-1936)
 |
Berkeley Moynihan was born on the island of Malta, the only son of a distinguished army captain. He received his premedical education at the Royal Naval School and his medical training at the Leeds Medical School (1885) and at the University of London (1887). In 1893, he was awarded a gold medal in the examination for master of surgery. In 1895, he married the daughter of T. R. Jessop, the man who preceded him as surgeon to the Leeds General Infirmary. Moynihan was a masterful surgeon, particularly for surgery of the abdomen. He served as professor of surgery from 1902, becoming emeritus professor in 1926. In 1905, he published his outstanding book, Abdominal Operations, which ran through four editions. Among his numerous contributions and distinctions were founder and editor of the British Journal of Surgery, president of the Royal College of Surgeons, Hunterian Professor, and successively, knight, baronet, and baron.
THOMAS KENNEDY DALZIEL (1861-1924)
 |
T. Kennedy Dalziel (pronounced “dee-yell”) was born in Scotland at Merkland, Penpont, Dumfriesshire. He received his early education at a private school in Dumfries and studied medicine at Edinburgh University, graduating in 1883. He continued his medical studies in Berlin and Vienna, where he specialized in experimental surgery and pathology. In 1885, he began his practice in Glasgow, and in 1889, he joined the staff of the Royal Hospital for Sick Children. For his services in World War I to the Advisory Council of the Royal Army Medical Corps, the king conferred on him the honor of knighthood. His successes and the public position he attained were the result of an unusual combination of qualities—charm, kindliness, extraordinary teaching skills, and marvelous manipulative dexterity. He was considered the best technical surgeon in the West of Scotland. His contributions to the medical literature were considerable, dealing primarily with that of abdominal surgery.
RICHARD H. MARSHAK (1912-1982)
 |
Richard Marshak received his MD with honors from the University of Louisville in 1937. After completing residencies in pathology and radiology, Marshak was invited by Burrill Crohn at Mount Sinai Hospital to join his private practice as a consulting radiologist. Consequently, Marshak saw hundreds of patients with gastrointestinal disorders. His unique forte consisted of correlating the pathologic findings of the gastrointestinal tract with the radiologic features. He, along with Bernard Wolf, elucidated the radiologic changes in esophagitis, hiatal hernia, and inflammatory bowel disease. Wolf’s collaborations, over a period of many years with Richard Marshak and Mansho Khilnani, on the physiologic and anatomic details of the esophagus and gastrointestinal tract and on the various aspects of inflammatory bowel disease, were unique. Much of what we take for granted today was first articulated during this era by these three men. Bernard Wolf, chairman of the department of radiology presented the Jacobi Medallion of the Alumni Association to Richard Marshak in 1972. Dick, as he was called, was the first president of the Society of Gastrointestinal Radiologists (SGR) and helped found the Health Insurance Plan. Before it became such a hot button, current topic, he pressed for the availability of medicine to everyone. He was the recipient of the Townsend Harris medal, given by the Alumni Association of the City College for outstanding achievements. He also received the Gold Medal Award from the Radiological Society of North America for distinction as an author, scholar, teacher, and scientist. Marshak was past president of the New York Academy of Gastroenterology, the New York Roentgen Society, and the American College of Gastroenterology. A dominant figure in radiology for more than 30 years, Richard H. Marshak belongs to a group of Mount Sinai physicians who are remembered equally for their scientific achievements and for their colorful personalities. Marshak finished his career as clinical professor of radiology at the Mount Sinai School of Medicine, continuing to work steadily until his death in 1982. As a founding member and the first president of the former Society of Gastrointestinal Radiologists, Marshak is recognized for his spirit of leadership and dedication to the SGR. An annual award given in his memory, the Richard H. Marshak International Lecture is presented to a member of the Society of Abdominal Radiology who represents the organization at the International Education Conference held annually in a country that cannot support education in the field of abdominal radiology. An annual contribution of $4,000 is received from the Marshak Fund in support of this award.
When the ravages of diabetes resulted in Marshak’s almost complete loss of vision, with the assistance of his long-time associate, Daniel Maklansky, himself a gifted radiologist and teacher, Marshak continued to give lectures, describing in detail slides he could barely see. Richard Marshak died of a heart attack at Lenox Hill Hospital, December 20, 1982, at the age of 70. He died just before the publication of the last in a series of books he had written on gastrointestinal diseases. (Photograph courtesy of the Mount Sinai Archives.)
Another observation involves the identification of genes associated with IBD. Pokorny and associates identified a genetic and clinical association between the DNA repair gene, MLH1, and both ulcerative colitis and Crohn’s disease (see Chapter 29).412 Most exciting is the identification of the NOD2 gene (now renamed CARD15) as the IBD1 gene in the pericentromeric region of chromosome 16 which signals the opening of a vast arena of genetic research to provide a basic understanding of IBD.217,251,389 NOD2 is involved in the activation of nuclear factor-kappa B (NF-κB) transcription factor that plays a significant role in Crohn’s disease. NOD2 encodes a protein homologous to plant disease resistance genes that are involved in the immune response to infectious organisms, particularly the leucine-rich region that binds to bacterial lipopolysaccharides. Ten percent of Crohn’s patients have a frameshift mutation (via a cytosine insertion) that fails to induce NF-κB in the presence of bacterial lipopolysaccharide, suggesting a common link to the failure of immune response to bacterial components and thereby possibly explaining the role of antibiotics or the value of probiotics in the therapy of Crohn’s. Certainly, Crohn’s disease appears to be influenced by a wide range of genetic and environmental factors.522
Signs, Symptoms, and Presentations
Patients with Crohn’s disease may present with very minimal symptoms and moderate complaints, or they may have fulminant manifestations. The considerable overlap in the presentations of ulcerative colitis and Crohn’s disease is addressed in Chapter 29. For example, individuals with Crohn’s disease may bleed, but this is not as frequent a presentation and may not be as severe.349 However, life-threatening hemorrhage has been reported.85 Abdominal pain may be mild or absent in patients with ulcerative colitis, but those with Crohn’s disease frequently complain of pain. This may be colicky in nature and may be associated with intestinal obstruction, or it may be continual and related to the presence of a septic process within the abdomen. An abdominal mass is not uncommonly found on physical examination in a patient with Crohn’s disease, but it is never seen in a patient with ulcerative colitis.
Diarrhea is usually a more troublesome concern in patients with ulcerative colitis. This may be because distal disease
tends to be associated with more urgency, and in some cases, tenesmus. Patients with Crohn’s disease may have rectal sparing and are less likely to experience this urgency. Conversely, with rectal involvement, Crohn’s patients experience symptoms similar to those with ulcerative colitis. It is important to consider also the possibility of opportunistic infections and concomitant neoplasms, including cytomegaloviral infection and Kaposi’s sarcoma, especially in individuals with prolonged immunosuppressive therapy for this disease (see later, Medical Management).89
tends to be associated with more urgency, and in some cases, tenesmus. Patients with Crohn’s disease may have rectal sparing and are less likely to experience this urgency. Conversely, with rectal involvement, Crohn’s patients experience symptoms similar to those with ulcerative colitis. It is important to consider also the possibility of opportunistic infections and concomitant neoplasms, including cytomegaloviral infection and Kaposi’s sarcoma, especially in individuals with prolonged immunosuppressive therapy for this disease (see later, Medical Management).89
Anal disease is much more commonly seen in patients with Crohn’s colitis than in those with ulcerative colitis. The presence of anal pain, swelling, and discharge may be a presenting feature of the former condition and may be the only abnormality observed on examination and on subsequent investigation. In the experience of the Lahey Clinic group with anal complications in patients with Crohn’s disease, 22% of 1,098 were so afflicted.585 Anal fissure was diagnosed in 29% of patients who had anal manifestations; a fistula was found in 28%, an abscess in 23%, and multiple presentations in 20%. Crohn’s colitis was much more frequently associated with an anal lesion than was Crohn’s disease of the small bowel (52% vs. 14%). Within 1 year following the anal manifestation, Crohn’s disease presented elsewhere in 59% of patients. All the remaining developed gastrointestinal disease within 5 years. In the St. Mark’s Hospital experience, 34% of patients with small bowel Crohn’s disease had anal lesions, whereas 58% of those with colon disease were found to have anal involvement.321 Of 126 consecutive patients with perianal Crohn’s disease seen regularly in one outpatient clinic, 48% were diagnosed as having an abscess.332
A high level of suspicion should exist if the examiner notes characteristic edematous tags, blue discoloration of the skin, an eccentrically located fissure, a broad-based ulcer, a rigid or strictured canal, or an anal fistula, especially if the patient reports gastrointestinal symptoms. A clinical classification of perianal Crohn’s disease has been proposed by Hughes.248 The reader is referred to Chapters 13 and 14 for a discussion of the management of anal complications.
Fever is usually not a concern in patients with ulcerative colitis unless the patient is severely ill (e.g., toxic megacolon). However, in those with Crohn’s disease, a pyrexia is not uncommonly noted and is usually due to the presence of an intra-abdominal abscess or undrained septic focus. Nausea and vomiting are not frequently noted in either condition unless there is evidence of intestinal obstruction. Anorexia, weight loss, anemia, and general debility are associated with relatively long-standing or fulminant disease.
Disease in Children and Adolescents
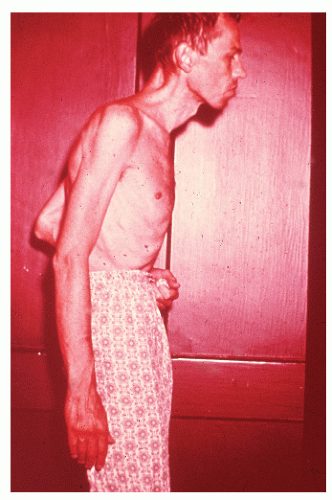
When the condition occurs in children, there may be a more rapid onset and progression than when the disease occurs in young adults. These youngsters often become chronically ill, have growth impairment, have decreased mental acuity, and are less developed physically than their healthy peers (see Figure 29-26). It is because of these concerns that implementation of either an elemental diet or parenteral nutrition is an especially important part of the management in this age group (see Medical Management).267,481 However, in order to be maximally effective, therapy must be initiated before puberty.267 Furthermore, unless medical treatment can achieve a sustained remission, operative intervention may be the only effective means for addressing the problem of retarded development (see later).119
Elliott and colleagues reported the prognosis of 57 patients with Crohn’s disease of the large bowel seen within 6 months of the onset of symptoms during 1969 to 1978 and followed until 1984 at St. Mark’s Hospital.133 The cumulative probability of an operation was 35% at 5 years and 39% at 10 years. They concluded that about one-half of all such patients can be treated successfully without abdominal surgery. However, children and adolescents with colonic disease are much more likely to require resection, often after a relatively short period of illness.171,432 Growth retardation as an indication for surgery may be one of the reasons for this difference.
As with adults, the efficacy of surgery for Crohn’s disease in children seems to depend mainly on disease location and perhaps the choice of surgical procedure itself.101 Assessment of growth and development, psychological support for both the patient and the family, and close cooperation between the physician and the surgeon are important concepts in the management of these young people.449,455,481
Physical Examination
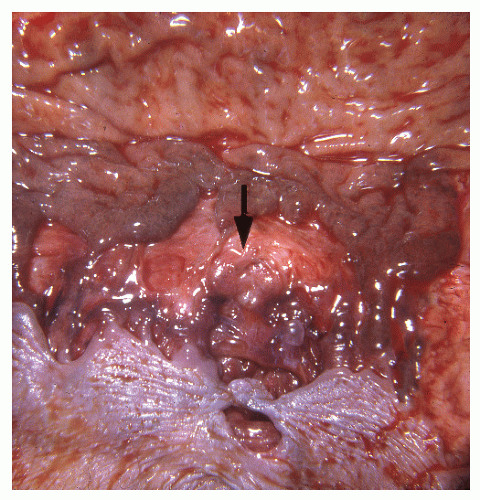
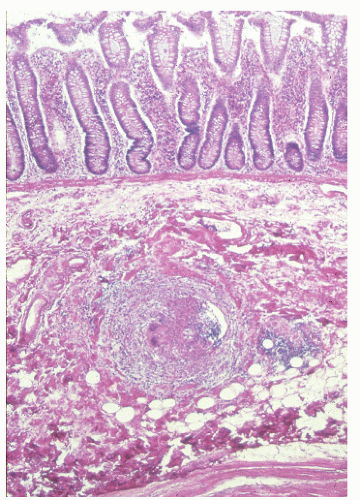
In contrast to individuals with ulcerative colitis, even in the absence of toxic megacolon, a patient with Crohn’s disease may demonstrate obvious findings on physical examination. As mentioned, although it is true that anorectal disease can occur with ulcerative colitis, it is much more common in those with Crohn’s disease. The diagnosis is often suspected on examination of the perianal skin (see Figure 14-51). Simple inspection will often show the edematous tags, fissures, abscess, or fistulas characteristically seen in this condition. The anal canal may be stenotic, fibrotic, and thickened on digital examination. If an anal fissure is apparent, severe pain is noted (Figure 30-1).
Pelvic examination in a woman may reveal a rectovaginal fistula, and bimanual examination may show the presence of a pelvic mass. A biopsy of a sinus tract or an abscess cavity may demonstrate the granulomas characteristic of Crohn’s colitis (Figure 30-2).
Pelvic examination in a woman may reveal a rectovaginal fistula, and bimanual examination may show the presence of a pelvic mass. A biopsy of a sinus tract or an abscess cavity may demonstrate the granulomas characteristic of Crohn’s colitis (Figure 30-2).
Abdominal findings are more common in patients with Crohn’s disease than in those with ulcerative colitis. A mass may be felt in the right iliac fossa, a common observation when regional enteritis involves the terminal ileum. A large, mesenteric abscess can often be palpated. Crohn’s colitis, however, usually is not associated with clinically demonstrable abdominal abnormalities.
Endoscopic Examination
Proctosigmoidoscopic examination is often helpful in differentiating between ulcerative colitis and Crohn’s disease. The rectum is always diseased during attacks of ulcerative colitis, whereas with Crohn’s colitis, 40% of patients have sparing of the rectum, irrespective of anal or perianal involvement (see Chapter 29). But when the rectum is involved by Crohn’s disease, differentiation between the two may be quite difficult.
A corollary to this observation is to perform biopsies distal to obvious inflammatory changes if the rectum appears to be spared because one may discover that the rectum is not truly normal. This may cause the physician to reassess the accuracy of a diagnosis of Crohn’s disease for what was initially thought to be lack of rectal involvement. Biopsy may be helpful because histologic changes suggestive of Crohn’s disease in particular may be apparent. Up to 20% of such patients may exhibit granulomata.
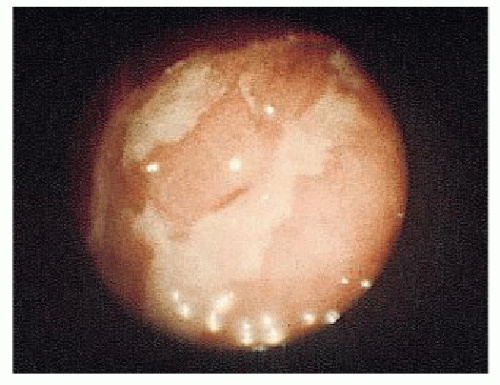
The place of colonoscopy in the evaluation and followup of IBD has been extensively reviewed by many authors. Teague and Waye recommend colonoscopy for five indications: differential diagnosis, resolution of radiographic abnormalities (e.g., filling defects and strictures), preoperative and postoperative evaluation in Crohn’s disease, examination of stomas, and screening for premalignant and malignant changes.546 With granulomatous colitis, Waye observed the following major colonoscopic findings: a normal rectum (obviously this is not always the case), asymmetry or eccentricity of involvement, cobblestone appearance, normal vasculature, edema of the bowel wall (as seen in ulcerative colitis), normal mucosa intervening between areas of ulceration, serpiginous ulcers (these may course for several centimeters), pseudopolyps (as in ulcerative colitis), and skip areas (lack of continuity of involvement).330 He adds another observation, the presence of amyloidosis in the biopsy specimen. An endoscopic index for determining the severity of colonic Crohn’s disease has also been proposed.344 Figure 30-3 illustrates the characteristic longitudinal ulcerations seen in Crohn’s disease.
Unfortunately, there are frequent difficulties in the interpretation of the biopsies obtained by means of proctosigmoidoscopy or colonoscopy. In a prospective study that Geboes and Vantrappen performed over an 18-month period, 71 colonoscopies were undertaken on 59 patients with Crohn’s disease.172 In comparison with barium enema examination, the segmental nature of the involvement was more apparent by means of colonoscopy. Microulceration was also more evident than by radiologic means. Radiographs yielded more information about the haustra, especially in the right colon. Colonoscopy permitted a histologic diagnosis in 24% of patients, but granulomas were found in only 19 of 321 specimens (6%). In more than one-fourth, the entire colon could not be examined, and no complications occurred. Hogan and colleagues observed that inconsistencies are often noted between macroscopic observations by the endoscopist and histologic interpretation of the biopsy specimen by the pathologist.242 They felt that the reason for this problem is the overlapping of the histologic features of the two conditions, ulcerative colitis and Crohn’s disease.
Changes in patients with ulcerative colitis are truly nonspecific, unless atypia or frank carcinoma supervenes. The most useful lesion found on colonoscopic mucosal biopsy of patients with Crohn’s disease is a granuloma. The limiting factor in establishing the correct diagnosis by means of biopsy, however, is the small size of the specimen. Hence, the physician or surgeon, having the benefit of clinical evaluation and the history, is often in the better position to make the correct diagnosis.
Distribution
The primary locations of the disease have been categorized by Bernell and coworkers as follows38:
Orojejunal (oral to the ligament of Treitz)
Small bowel (excluding terminal 30 cm of ileum)
Ileocecal (distal 30 cm of ileum with or without cecal involvement)
Continuous ileocolic (continuous ileocolic involvement)
Discontinuous ileocolic (both small and large bowel involvement, but without continuous inflammation in the ileocecal region)
Colorectal (confined to the colon or rectum or both only)
Radiographic Features
As previously mentioned, Crohn’s disease can occur anywhere in the alimentary tract. The disease tends to be segmental and asymmetric. Radiologic findings include skip lesions, contour defects, longitudinal ulcers, transverse fissures, eccentric involvement, pseudodiverticula, narrowing or stricture formation, pseudopolypoid changes that may be cobblestone-like, sinus tracts, and fistulas.341
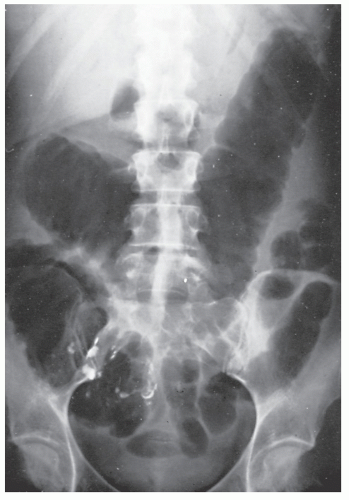
A plain film of the abdomen may be quite useful in the early stages of Crohn’s colitis. Although toxic megacolon is much less common in Crohn’s disease than it is in ulcerative colitis, acute toxic dilatation may occur before any firm cicatrix has formed in the bowel wall (Figure 30-4).
Colon
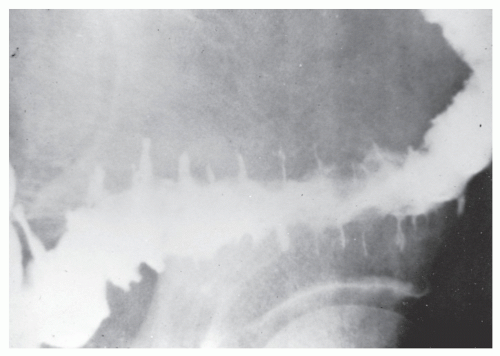
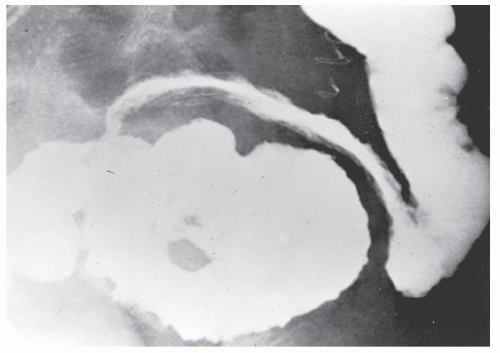
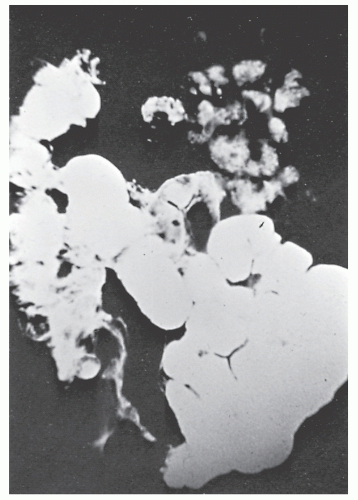
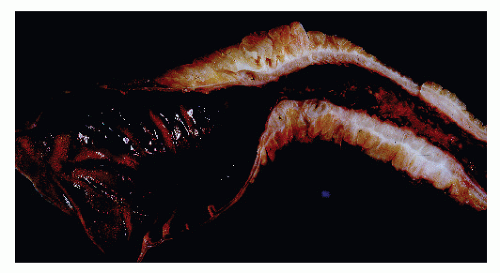
The postevacuation film is most useful in identifying numerous discrete ulcers (Figure 30-5). Small ulcerations may combine to produce large, longitudinal ulcers (Figure 30-6), and when the longitudinal ulcers combine with transverse fissures, they produce the cobblestone appearance seen radiologically (Figure 30-7). Intramural fistulas can result from the coalescing of the numerous longitudinal ulcers, which in turn may produce a doublelumen appearance (Figure 30-8). Ulcerations may penetrate beyond the contour of the bowel and present as numerous long spicules or as sinus tracts (Figure 30-9). These deep fissures may be confused with diverticula, but with experience the physician should be readily able to differentiate them. If one examines the whole-mount specimen shown in Figure 30-42, one can appreciate how such a radiologic picture can evolve.
Although the standard barium enema examination has been routinely employed in the past for evaluating IBD, contemporary evidence suggests that the air-contrast technique is preferred. Radiologists have prided themselves on their ability to identify somewhat unusual radiographic features of IBD. These include mucosal bridging and aphthoid ulcers.44,216,477,513 Although these findings are helpful in the evaluation of patients with IBD, their ready documentation by means of colonoscopy would diminish the value of the radiologic observation.
Superficial mucosal abnormalities are not uncommonly seen in the distal part of the ileum, and concern is often
expressed as to the likelihood of such a patient developing clinically recognizable Crohn’s disease. Ekberg and colleagues identified mucosal abnormalities in the distal ileum of 21 patients by means of air-contrast enemas.131 From 4 to 7 years later, neither Crohn’s disease nor any other progressive condition of the small bowel developed.
expressed as to the likelihood of such a patient developing clinically recognizable Crohn’s disease. Ekberg and colleagues identified mucosal abnormalities in the distal ileum of 21 patients by means of air-contrast enemas.131 From 4 to 7 years later, neither Crohn’s disease nor any other progressive condition of the small bowel developed.
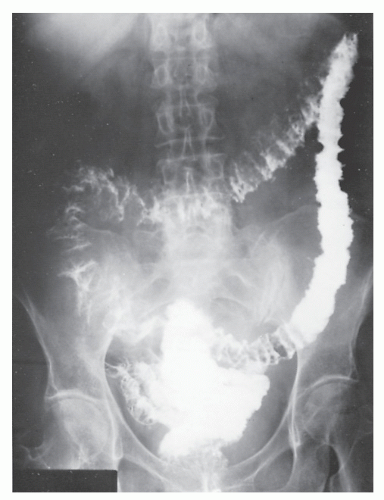
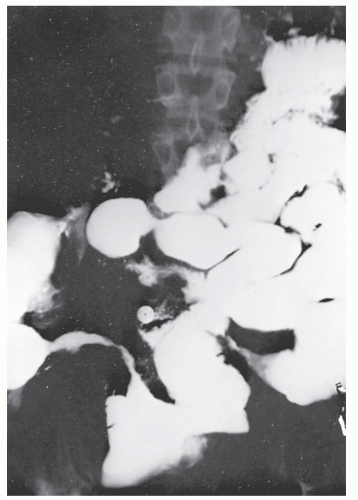
Figure 30-10 demonstrates a number of the classical changes that one may see in the radiographic appearance of Crohn’s colitis. These include segmental distribution with sparing of the rectum, stenosis, thickening of the bowel wall, ulceration, and a suggestion of a double-lumen appearance.
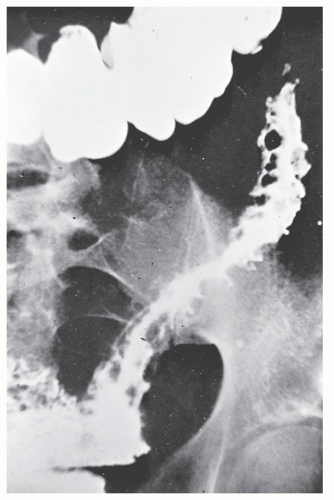
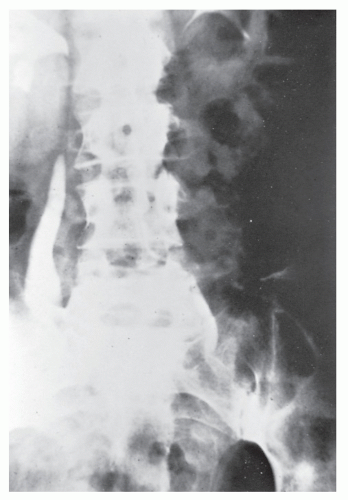
Strictures are of variable lengths and may be quite extensive indeed (Figures 30-11 and 30-12). When a stricture occurs in Crohn’s disease, it does not imply a malignant association, such as when it is seen in a patient with ulcerative colitis. However, individuals with Crohn’s disease have been shown to have an increased risk for the development of malignancy (see Relationship to Carcinoma). Differential diagnosis between a Crohn’s stricture and that of a carcinoma is usually not difficult. Close inspection often reveals that the bowel in adjacent areas is ulcerated (Figure 30-13). Contrast this x-ray with that of Figure 30-14. Although a scirrhous carcinoma must always be considered in the differential diagnosis, the lack of associated ulceration or inflammatory changes elsewhere in the colon usually clarifies the dilemma. Still, one must be wary of the possibility (see Figure 30-12).
A most difficult problem is to differentiate radiographically Crohn’s disease from tuberculosis (see Chapter 33). When the condition is confined to the ileocecal region, it is virtually impossible to distinguish between the two diseases.
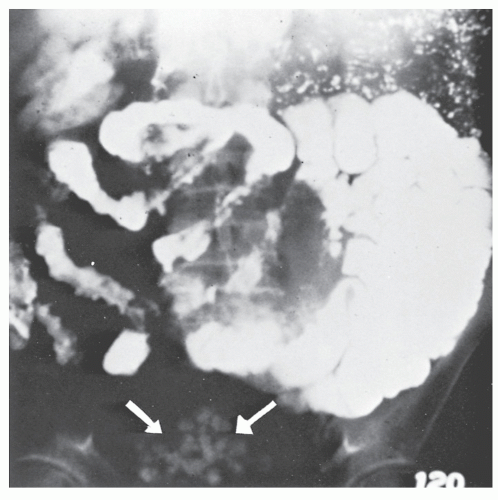
Barium enema has been used to evaluate the anal canal in patients with Crohn’s disease.120 Although direct visual examination is more accurate, there are characteristic changes that may be identified by means of careful radiologic examination of the area. The hallmark of a radiologically normal anal canal is the presence of straight, smooth lines of barium between the folds, whereas an abnormal anal canal may show distortion of the folds, ulcers, fissures, sinus tracts, and fistulas.120 However, if one requires the assistance of a radiologist to make the diagnosis of anal Crohn’s disease, that physician must be considered diagnostically destitute.
Small Bowel
Upper gastrointestinal and small bowel x-ray films are quite helpful for evaluating IBD in this area of the alimentary tract, especially because there is no truly adequate nonoperative endoscopic examination of the small intestine, except for the very distal ileum, putting aside capsule endoscopy. Radiologically, evaluation of the terminal ileum is best obtained by reflux on barium enema examination, but unfortunately, as many as 20% of patients will not demonstrate this phenomenon. Alternatively, a small bowel follow-through study or an enteroclysis with good spot films can be used.
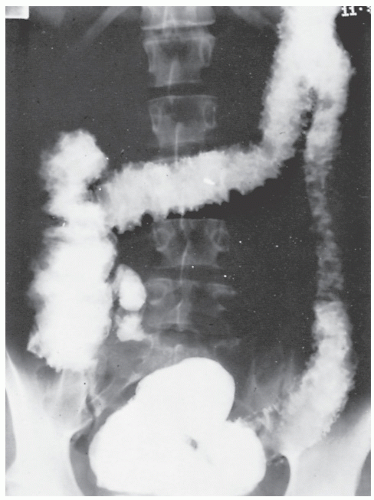
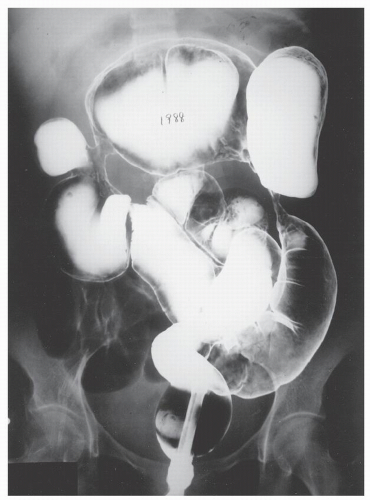 FIGURE 30-12. Air-contrast barium enema reveals multiple strictures in the colon in an individual with Crohn’s disease. At the time of surgery, these all proved to be benign. |
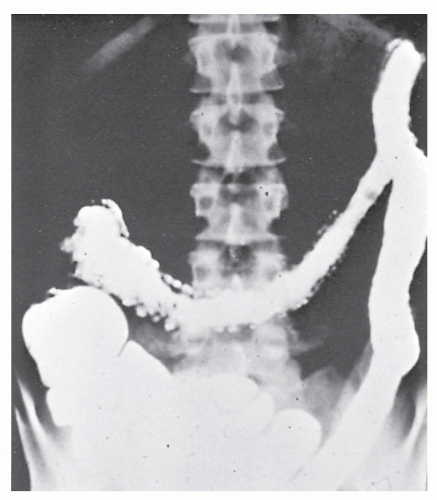
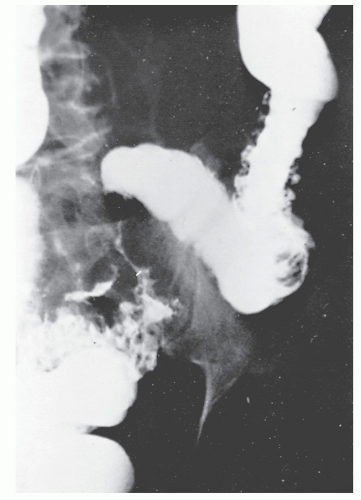
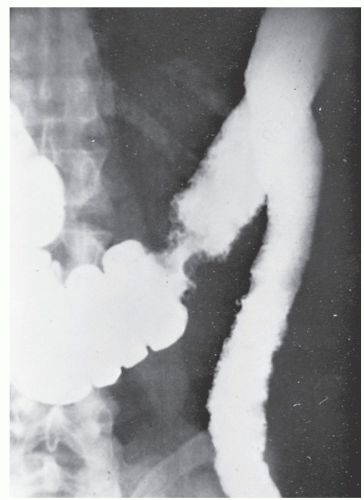
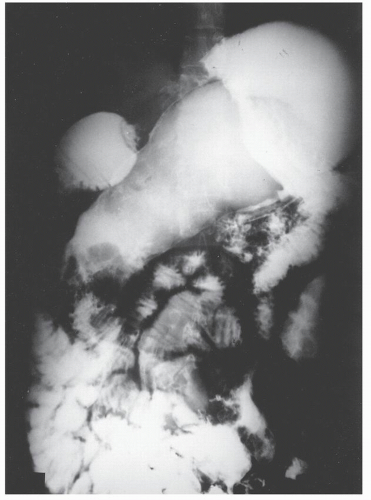
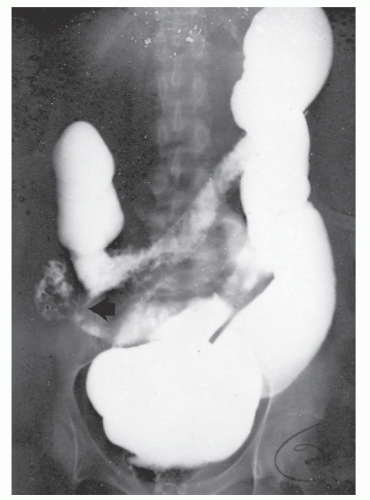
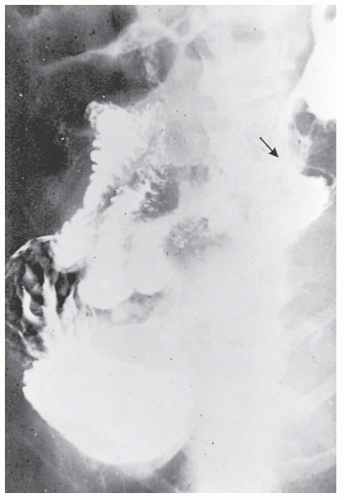
Increasingly, CT enterography has become utilized for demonstrating luminal disease, extent of involvement, points of obstruction, and extraintestinal complications. This study will also show the degree of mural thickening, the presence of fistula, as well as sepsis/abscess. It may show the site of target organ involvement, such as duodenum, sigmoid, and ileocolic fistula. This serves as a guide to the endoscopist as well. In the case of ileosigmoid fistula, this may be confirmed endoscopically by the appearance of a granulomatous nodule, wherein the sigmoid and rectum are otherwise normal. This may, in the case of a rare entity, ileorectal fistula, have a bearing on the surgical strategy—such as the need for mobilization of the rectum when segmental rectal resection may be required. Crohn’s disease of the terminal ileum has a characteristic appearance. Thickening of the bowel wall narrows the lumen, resulting in a degree of obstruction in some patients. This is the most frequent cause of abdominal pain in individuals with Crohn’s disease. The radiologic appearance of the terminal ileum has been described as having a “string sign” (Figure 30-15). Involvement of the terminal ileum may be seen as an isolated finding or may be associated with multiple diseased areas throughout the small intestine (Figure 30-16). In contrast to radiologic evaluation of the colon for Crohn’s disease, it is virtually impossible to differentiate a benign from a malignant stricture in the small intestine (Figure 30-17). As with carcinoma of the small bowel in an individual without Crohn’s disease, the prognosis is extremely poor.
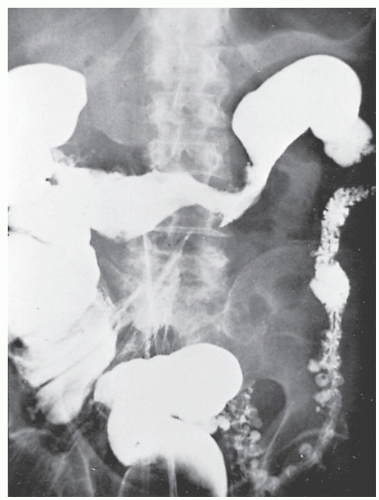
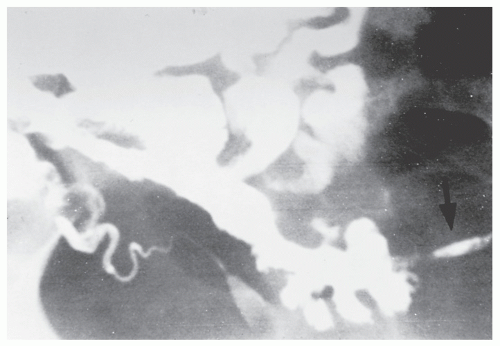
Fistulous complications are frequently seen in patients with Crohn’s disease. Communication between the ileum and colon is not uncommon (Figure 30-18), but other types of fistulas have been observed, including coloduodenal (Figures 30-19 and 30-20), and those to pelvic organs (Figure 30-21).
Ultrasound
Ultrasound examination is of quite limited value in patients with IBD because of the presence of considerable artifact associated with the loops of bowel. The presence of air or fluid in the intestine and adhesed loops may simulate a septic focus. That stated, Sonnenberg and colleagues performed a prospective clinical trial comparing 51 patients with Crohn’s disease with 124 controlled subjects by means of grayscale ultrasound.528 Diagnosis by ultrasound reflected primarily the thickening of the gastrointestinal wall itself, perceived as a characteristic “target” appearance. The study demonstrated that there were very few false negatives. The occasional false-positive phenomenon was usually attributed to the presence of a gastrointestinal tumor.
van Outryve and coworkers studied transrectal ultrasound in individuals with Crohn’s disease and in control subjects.571 The authors observed that the procedure sharply delineates the rectal wall and may detect unsuspected abscesses and fistulas in the pararectal and paraanal tissues (see Chapters 5 and 7). Abdominal ultrasound may also allow for accurate measurement of mural thickness. In the case of multiple small bowel strictures, this may impact on surgical decision making. For example, one is more likely to favor resection of a stricture if the wall thickness is 9 mm or more. Strictureplasty, if performed under this circumstance, leads to early recurrence (see later).
Computed Tomography
Computed tomography (CT) is able to demonstrate thickening of the colon, nodularity, adenopathy, and intra-abdominal abscess (Figure 30-22). The presence of any fistula, especially an enterocutaneous communication, is often demonstrable by means of CT scan with oral or rectal contrast. In most cases, however, adequate evaluation of intra-abdominal pathology can be obtained by means of endoscopic examination and by standard contrast techniques.
Yousem and colleagues studied CT scans of 200 consecutive patients with Crohn’s disease in order to determine the frequency and patterns of perirectal and perianal involvement.609 They observed inflammation of fat planes (73%), bowel wall thickening (30%), fistulas or sinus tracts (22%), and abscesses (14%). Because more than one-third had abnormal CT manifestations below the symphysis pubis, the authors emphasize the importance of scanning sequences to the perineum in individuals with Crohn’s disease.
Gore and colleagues attempted to provide the perspective of CT criteria in the evaluation of ulcerative, granulomatous and indeterminate colitis.191 Unfortunately, features were often overlapping, and CT did not alter the original diagnosis in any patient.
Capsule Endoscopy
The application of capsule endoscopy for evaluating the small bowel has been discussed in Chapter 5. Several studies have been published that attest to its value in assessing the small bowel in individuals with Crohn’s disease.132,162,236 However, although it may be useful for identifying an occult source of bleeding, the reality is that the overwhelming majority of patients can have their disease identified by simpler, less expensive means. Furthermore, there is a real risk of precipitating a small bowel obstruction if the capsule cannot pass a strictured area. Still, in a limited number of individuals, capsule endoscopy may be a useful diagnostic tool for this condition.
Angiography
Another method for identifying the site of small bowel bleeding in an individual with Crohn’s disease has been described, that of the combined use of preoperative angiography and highly selective methylene blue injection.441 It was felt that this technique may aid the surgeon in the preoperative and intraoperative localization of occult bleeding sites in this condition.
The Significance of Special Laboratory Studies
A number of specialized laboratory studies have been advised, primarily for evaluation of Crohn’s disease. Some have suggested the use of an indium-labeled leukocyte scan to distinguish patients for whom medical therapy may be preferable from those who may be optimally treated by surgery.518 For example, in active Crohn’s disease, labeled leukocytes are excreted into the bowel lumen from the inflamed mucosa. Patients with positive scans, therefore, have higher values of indices of disease activity. In a study by Slaton and colleagues, a negative indium leukocyte scan suggested a fibrotic ileal stricture and the advisability of surgical intervention.518 As an anatomic indicator of acute granulocytic infiltration of the intestinal mucosa and submucosa, Nelson and colleagues found that this scan had a 97% rate of sensitivity and a 100% specificity.376 The study may be best applied in individuals with fulminant disease, especially those who cannot safely be put through the rigors of endoscopy or barium contrast radiologic evaluation.
Brignola and colleagues studied various laboratory indices to determine whether any had predictive value for recurrence of Crohn’s disease.58 There was a significant correlation with recurrence and alteration of acid
1-glycoprotein, 2-globulin, and erythrocyte sedimentation rate in comparison with the patients who remained in remission. Also, it has been demonstrated that patients with Crohn’s disease requiring operative treatment often have a severe peripheral lymphopenia.233 Mahida and colleagues have been able to detect interleukin-6 in seven out of eight peripheral and mesenteric samples from patients with Crohn’s disease.331 Heimann and Aufses showed that individuals who developed recurrences had significantly lower preoperative lymphocyte counts than those who were free of disease 3 years following resection.232 As more information becomes available, it is possible that these and other studies will help the physician and surgeon determine which patients are at increased risk and perhaps influence the timing and the type of therapy.
1-glycoprotein, 2-globulin, and erythrocyte sedimentation rate in comparison with the patients who remained in remission. Also, it has been demonstrated that patients with Crohn’s disease requiring operative treatment often have a severe peripheral lymphopenia.233 Mahida and colleagues have been able to detect interleukin-6 in seven out of eight peripheral and mesenteric samples from patients with Crohn’s disease.331 Heimann and Aufses showed that individuals who developed recurrences had significantly lower preoperative lymphocyte counts than those who were free of disease 3 years following resection.232 As more information becomes available, it is possible that these and other studies will help the physician and surgeon determine which patients are at increased risk and perhaps influence the timing and the type of therapy.
Pathology
Macroscopic Appearance
Crohn’s disease may have protean clinical and pathologic manifestations. The condition can be confined to the colon alone or may involve only the anal canal. Fistulas, segmental involvement, rectal sparing, perianal disease, and abscess formation are all characteristic of granulomatous colitis. Some of the earliest changes in the serosal aspect of the small intestine involved by Crohn’s disease may be immediately recognizable if the patient is submitted to surgery. The intraoperative recognition of Crohn’s disease can also be inferred by the corkscrew appearance of vessels on the serosal aspect. This implies that this segment has been subjected to intermittent obstruction. The serosal vessels elongate as the bowel distends proximal to a stricture, and
when the intermittent dilatation contracts, these stretched vessels assume a corkscrew appearance. Other points suggesting involved small bowel are mesenteric marginal thickening, which corresponds to the longitudinal ulcers of Crohn’s disease in the small bowel.
when the intermittent dilatation contracts, these stretched vessels assume a corkscrew appearance. Other points suggesting involved small bowel are mesenteric marginal thickening, which corresponds to the longitudinal ulcers of Crohn’s disease in the small bowel.
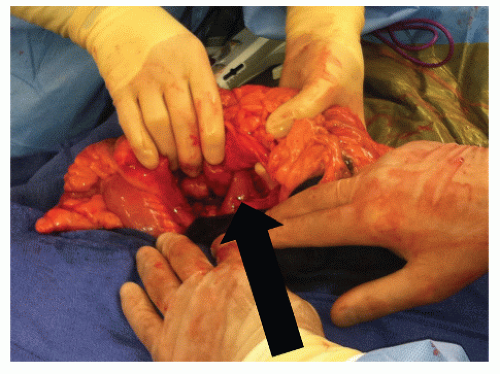 FIGURE 30-20. Coloduodenal fistula. At operation, fistula between the two structures is clearly demonstrated (arrow). |
Subserosal extension of fat around the surface of the bowel (“fat wrapping”) and a prominent vascular pattern in the serosa are characteristic of the disease (Figure 30-23). The serosal surface may be granular and bleed easily on any intraoperative abrasion. It has been demonstrated that fat wrapping correlates best with transmural inflammation and represents part of the connective tissue changes that accompany intestinal Crohn’s disease.504
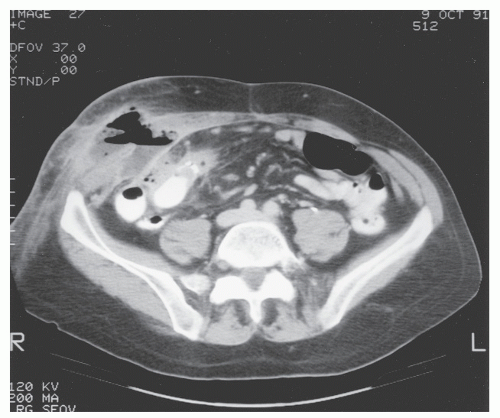 FIGURE 30-22. Computed tomography demonstrates abdominal wall abscess on the right side. This was secondary to a perforating ileocolic Crohn’s inflammation. |
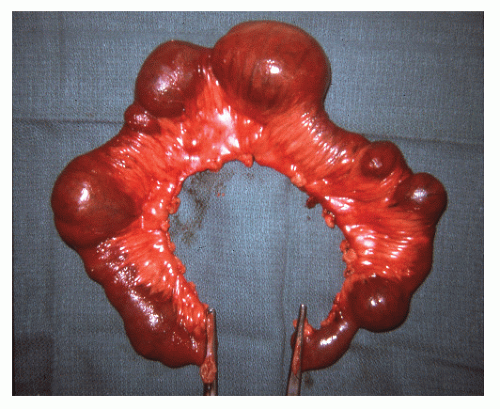
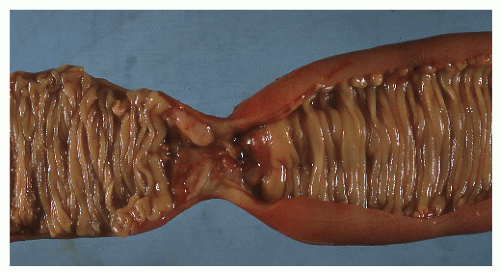
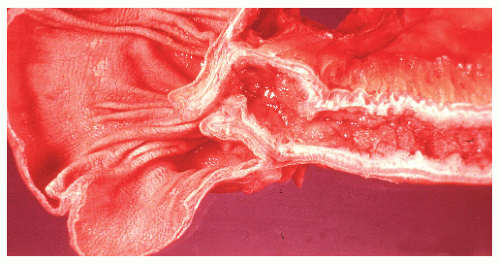
The disease frequently affects the bowel in a segmental fashion. This may produce extensive skip areas (Figures 30-24 and 30-25), limited involvement to an area of the bowel (Figure 30-26), or even a focal, isolated stricture (Figure 30-27).
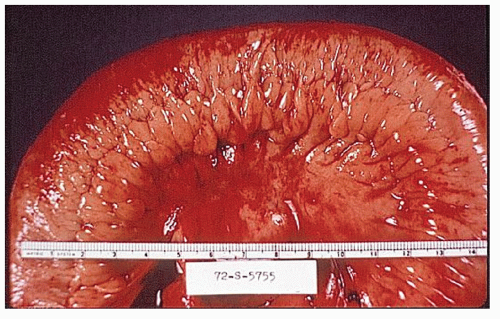
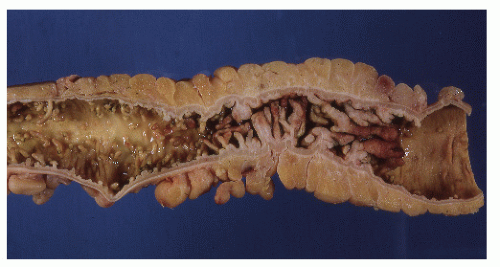
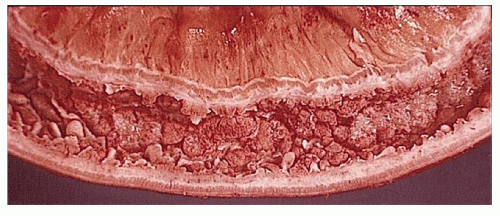
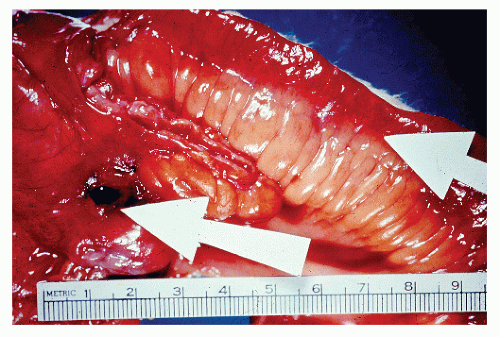
Classically, Crohn’s colitis involves the intestine in an asymmetric fashion. Areas of the bowel may demonstrate disease on the mucosal aspect with sparing of adjacent sites, leaving islands of somewhat edematous but otherwise nonulcerated mucosa (Figures 30-28,30-29 and 30-30). Ulceration in an irregular fashion with large areas of uninvolved mucosa interspersed between broad, twisting lesions is quite characteristic. The relative sparing between ulcers is not seen in ulcerative colitis. Another characteristic feature of the macroscopic appearance of Crohn’s disease is the thickening of the bowel wall. Involvement through all the layers, along with the cobblestone appearance of the mucosa, has been described as “stones in a running brook” (Figure 30-31).
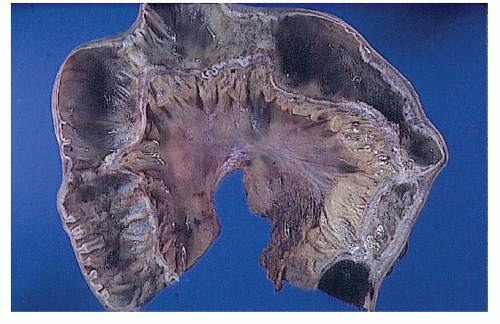 FIGURE 30-25. Crohn’s disease. This opened specimen from Figure 30-24 reveals the segmental nature of the disease. Thickening of the mesentery is a prominent feature. (From Corman ML, Veidenheimer MC, Nugent FW, et al. Diseases of the Anus, Rectum and Colon. Part II: Non-specific Inflammatory Bowel Disease. New York, NY: Medcom; 1976.) |
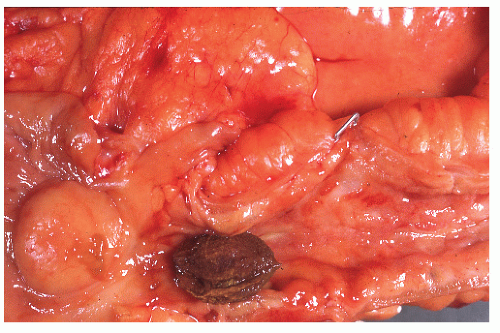
Crohn’s colitis frequently involves the colon and ileum in continuity. Conversely, cecal ulceration can be seen with primarily ileal disease (Figure 30-32). Occasionally, the ileal disease may terminate abruptly at the ileocecal valve, sparing the large bowel (Figure 30-33). Thickening of the bowel wall may produce sufficient narrowing to precipitate intestinal obstruction or to impede the passage of swallowed seeds or nuts (Figure 30-34). Gallstone ileus has even been reported to produce obstruction at a point of stenosis caused by Crohn’s disease.499
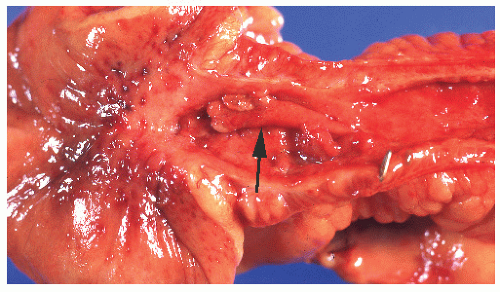
A common manifestation of Crohn’s disease is fistula formation. Fistulas may occur into any adjacent organ, such as the small or large bowel, bladder, vagina, uterus, ureter, or skin. Burrowing of the fissures deep into the bowel wall predisposes to fistula formation. Fistulas occur more commonly in the mesocolic aspect of the bowel than on the antimesocolic border (Figure 30-35).
Although nodal adenopathy is frequently present, the location of enlarged, paraileal lymph nodes is a reasonably accurate way of identifying the proximal extent of disease without the need of verifying this by entering the bowel.
Occasionally, diffuse mucosal disease may produce a pseudopolypoid pattern similar to that of chronic ulcerative colitis. Giant pseudopolyps may actually mimic neoplasms endoscopically and radiographically. The condition is most likely the result of fusion of numerous fingerlike pseudopolyps.207 A number of reports and reviews of this manifestation have been published.113,207,263
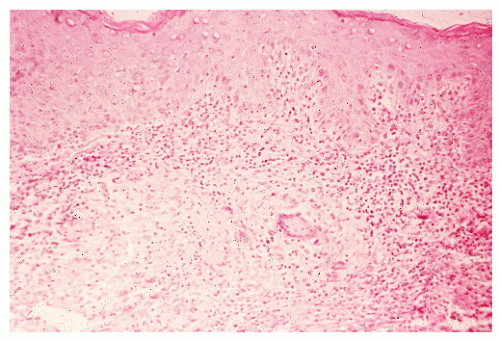
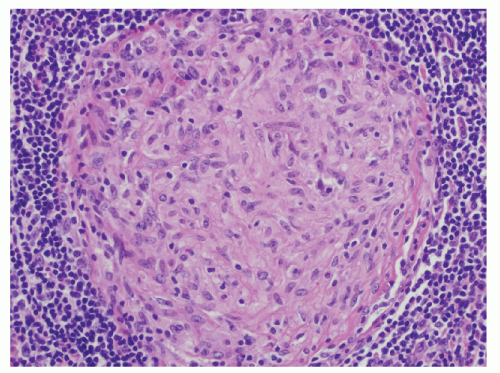
Histologic Appearance
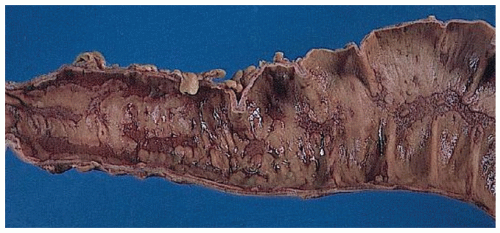
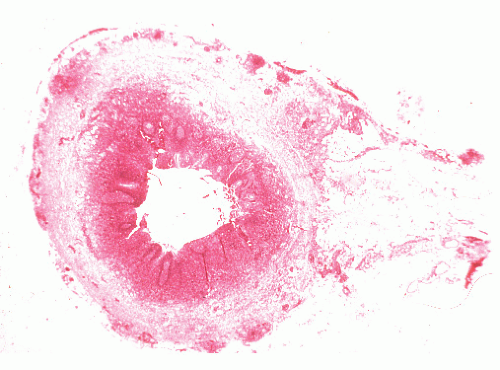
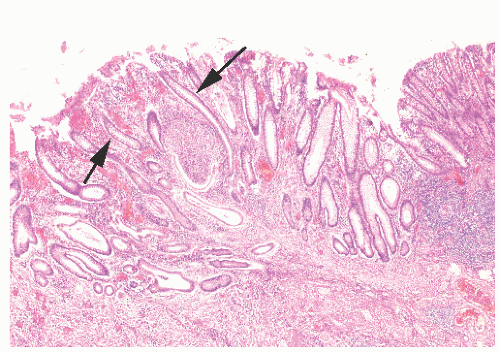
The three primary histopathologic findings in patients with Crohn’s colitis are transmural inflammation and fibrosis, granulomas, and narrow, deeply penetrating ulcers or “fissures” (Figure 30-36). The mucosal inflammation of Crohn’s disease differs from that of ulcerative colitis in that typically there are fewer crypt abscesses, there is less congestion, and there is better preservation of the goblet cell population (Figure 30-37).
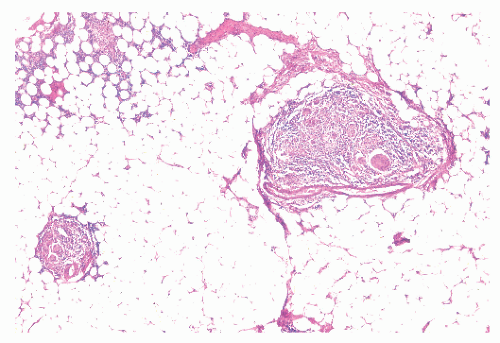
Granulomas may occur in any part of the bowel wall and are usually identified in approximately two-thirds of all patients with Crohn’s colitis. If a biopsy is performed in an attempt to differentiate between the two inflammatory conditions, the material should be obtained from a noninflamed area if possible (Figure 30-38). A granuloma, albeit a foreign body type, may actually be seen even with ulcerative colitis in an area of acute inflammation. Multiple biopsy specimens are suggested because submucosal lesions tend to be very small (microgranulomas).289 The microscopic appearance of
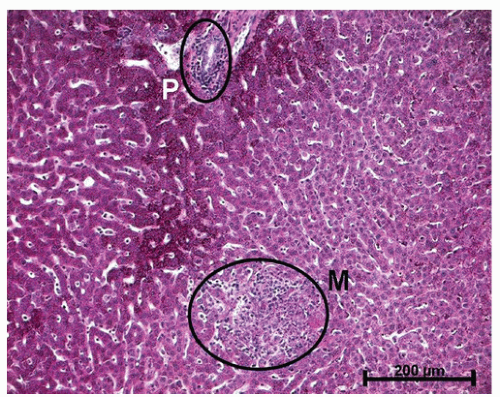
the granuloma is not diagnostic, and the possibility of an infectious agent should always be considered (Figure 30-39). Granulomas can also occur in the liver (Figure 30-40) and in the omentum (Figure 30-41) as well as in other sites.
the granuloma is not diagnostic, and the possibility of an infectious agent should always be considered (Figure 30-39). Granulomas can also occur in the liver (Figure 30-40) and in the omentum (Figure 30-41) as well as in other sites.
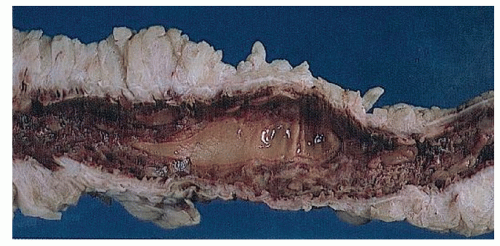
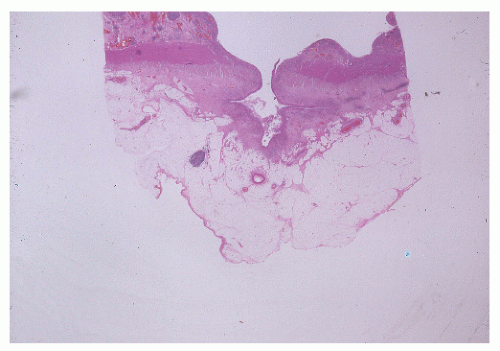
Narrow, deeply penetrating ulcers or fissures are the third characteristic feature of Crohn’s disease. The fissures may penetrate through the inner circular layer of the muscularis and are visible on the radiographs after a barium enema as spicules. A sinus tract present in the fat adjacent to the bowel wall indicates that one of the fissures has penetrated through the wall (Figure 30-42). When this occurs, a sinus may burrow into another organ to produce a fistula.
Information about the pathology of IBD has been further gleaned by means of electron microscopy. Early epithelial changes can be identified using this modality in areas that appear to be uninvolved. These include necrosis of individual columnar epithelial cells; budding of the tips of microvilli; thickening, shortening, irregularity, and fusion of intestinal villi; numerous Paneth’s cells; hyperplasia of goblet cells; and augmented mucous secretion.271 Other studies such as tissue-enzyme analysis, jejunal-surface pH, and differences in sodium flux and mucosal potential imply that the disease often is far more extensive than is recognized by other, more conventional means, and certainly much more extensive than is usually apparent at the time of surgery.
Another observation is the increased secretion of mucus by the bowel in Crohn’s disease as compared with decreased colonic mucus in ulcerative colitis. The decrease may be explained by destruction of the epithelial cells.271 A number of biochemical changes have also been observed.
Extraintestinal Manifestations
For convenience and in order to avoid duplication, I have elected to place the discussion of extraintestinal manifestations in this chapter. Moreover, because so many of the
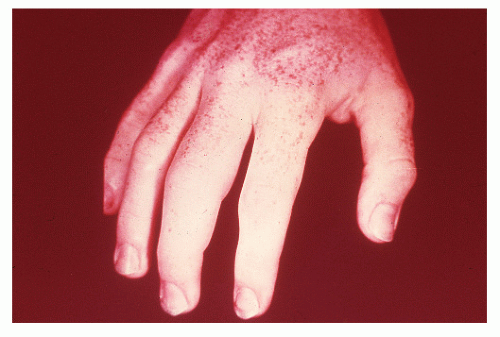
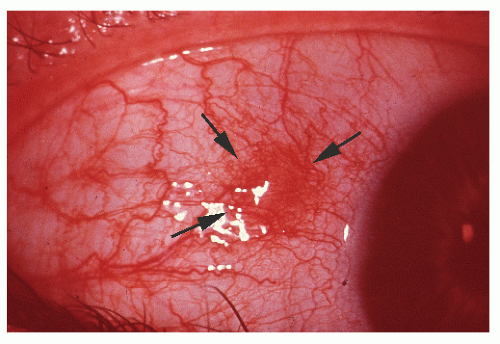
manifestations in other areas of the body are exclusively seen in Crohn’s disease, the discussion is placed here, although many such problems may be seen in both ulcerative colitis and Crohn’s disease. Increasing evidence supports the statement that inflammatory disease of the intestine is a systemic problem rather than one localized to the small or large bowel. In a population-based study from Sweden of 1,274 patients with ulcerative colitis, the overall prevalence of extracolonic diagnoses was 21%.363 As discussed in Chapter 29, many etiologic concepts have been considered, but regardless of the sequence of pathologic changes in the colon, there is little question about the presence of related events, at times profound, in distant areas of the body. The joints, skin, liver, kidneys, eyes, mouth, blood, nervous system, and, of course, other areas of the alimentary tract may be sites of lesions that, at least in the extraintestinal manifestations, often seem dependent on the presence of diseased bowel. So broad indeed is the spectrum of Crohn’s disease that specialists in dentistry, otorhinolaryngology, ophthalmology, and dermatology must be prepared to recognize its manifestations.
manifestations in other areas of the body are exclusively seen in Crohn’s disease, the discussion is placed here, although many such problems may be seen in both ulcerative colitis and Crohn’s disease. Increasing evidence supports the statement that inflammatory disease of the intestine is a systemic problem rather than one localized to the small or large bowel. In a population-based study from Sweden of 1,274 patients with ulcerative colitis, the overall prevalence of extracolonic diagnoses was 21%.363 As discussed in Chapter 29, many etiologic concepts have been considered, but regardless of the sequence of pathologic changes in the colon, there is little question about the presence of related events, at times profound, in distant areas of the body. The joints, skin, liver, kidneys, eyes, mouth, blood, nervous system, and, of course, other areas of the alimentary tract may be sites of lesions that, at least in the extraintestinal manifestations, often seem dependent on the presence of diseased bowel. So broad indeed is the spectrum of Crohn’s disease that specialists in dentistry, otorhinolaryngology, ophthalmology, and dermatology must be prepared to recognize its manifestations.
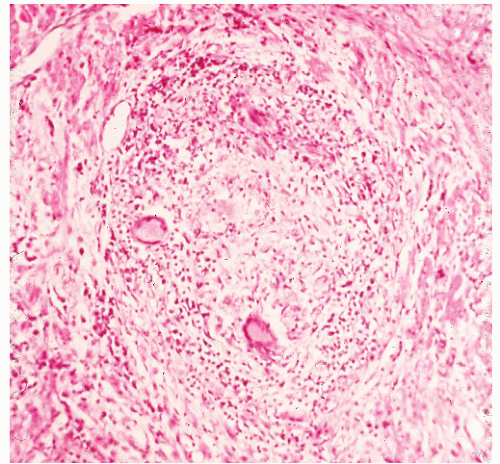 FIGURE 30-40. Crohn’s disease. Granuloma of the liver in a patient with Crohn’s colitis. (Original magnification × 260.) |
Oral Manifestations
Oral lesions were first identified in Crohn’s disease by Dudeney and Todd in 1969.121 Since then, a number of papers
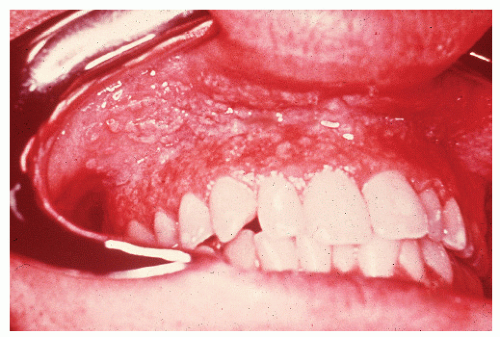
have been published on the subject.42,47,521,574 Inflammatory changes in the mouth may even be the initial site of involvement.87 Basu and Asquith reviewed the oral manifestations of IBD, describing a number of lesions.33 These included recurrent aphthous ulcers, pyoderma gangrenosum, pyostomatitis vegetans, hemorrhagic ulceration, glossitis, macroglossia, and moniliasis. The authors reported that the incidence is not as uncommon as one might expect, with up to 20% having been described as having oral lesions. The most frequently affected areas and their respective appearances are the buccal mucosa (a cobblestone pattern), the vestibule (linear, hyperplastic folds), and the lips (diffusely swollen and indurated).43
have been published on the subject.42,47,521,574 Inflammatory changes in the mouth may even be the initial site of involvement.87 Basu and Asquith reviewed the oral manifestations of IBD, describing a number of lesions.33 These included recurrent aphthous ulcers, pyoderma gangrenosum, pyostomatitis vegetans, hemorrhagic ulceration, glossitis, macroglossia, and moniliasis. The authors reported that the incidence is not as uncommon as one might expect, with up to 20% having been described as having oral lesions. The most frequently affected areas and their respective appearances are the buccal mucosa (a cobblestone pattern), the vestibule (linear, hyperplastic folds), and the lips (diffusely swollen and indurated).43
Aphthous ulcers usually parallel the course or activity of the IBD: the more active the disease, the more likely one is to develop this complication. Biopsy usually shows a chronic inflammatory reaction.
Pyostomatitis vegetans is an unusual manifestation of IBD. Papillary projections of mucous membrane can be seen separated by small areas of ulceration (Figure 30-43). Biopsy may reveal suprabasal separation of the oral epithelium and infiltration with eosinophils.
The recognition of the specific oral granuloma is important because it may be the first manifestation of Crohn’s disease.521 Scully and colleagues reported 19 patients with clinical evidence of oral Crohn’s disease but no intestinal symptoms.498 More than one-third were demonstrated either on rectal biopsy or by contrast gastrointestinal x-ray films to have IBD, even in the absence of symptoms.
Treatment
Because the lesions are resistant to local therapy, general measures for soothing the oral discomfort are advised. The symptoms and clinical findings of oral problems are often ameliorated with appropriate treatment of the intestinal disease.
Esophageal Involvement
Patients with Crohn’s disease of the esophagus will present with symptoms not unlike those associated with other lesions of that organ, such as carcinoma. Substernal discomfort, dysphagia, epigastric pain, weight loss, nausea, and vomiting are all part of the clinical spectrum. Other gastrointestinal symptoms are usually due to the presence of disease elsewhere in the alimentary tract.126,174,231 It must be remembered that dysphagia and the demonstration of an esophageal ulcer or esophagitis in a patient with known Crohn’s disease can be due to reflux esophagitis, certain drugs or corrosive agents, pressure from a nasogastric tube, infectious agents, sarcoidosis, or Behçet’s disease.380 In point of fact, many published reports of esophageal Crohn’s disease cannot be supported by critical review.
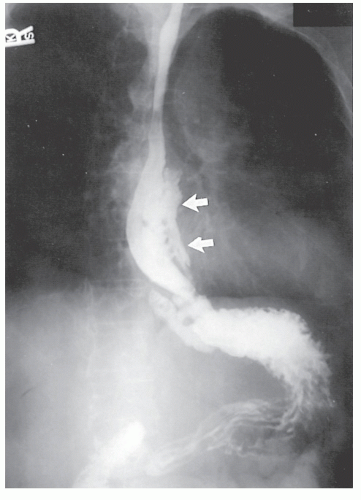
Physical examination is usually unrewarding with respect to esophageal involvement. Diagnosis is usually made by a high index of suspicion and radiologic investigation, which obviously would include a barium swallow (Figure 30-44). This study may reveal thickened mucosal folds, multiple ulcerations, or, most commonly, a stricture. This last finding makes differentiation from carcinoma quite difficult, except that the presence of disease elsewhere or the relatively young age of the patient should lead one to suspect an inflammatory process.
Endoscopic examination will usually reveal hyperemia with possibly either an ulcerated mucosa or the presence of an inflammatory stricture. Biopsies usually show an inflammatory reaction, but the absence of granulomata does not exclude the diagnosis of Crohn’s disease.126
Treatment usually consists of the standard medical management appropriate for Crohn’s disease of the small or large bowel (see Chapter 29 and Medical Management). Resection is rarely indicated.
Gastroduodenal Crohn’s Disease
Crohn’s disease involving the stomach and duodenum may not be as rare as originally suspected. Since the original description in 1937 by Gottlieb and Alpert of the condition in the duodenum, a number of cases have been reported.192 In 1981, Korelitz and colleagues performed random endoscopic biopsies of the stomach and duodenal mucosa in patients with Crohn’s disease, frequently demonstrating the presence of microscopic alterations consistent with this inflammatory process in the upper gastrointestinal tract.282 Clinically, evident IBD of the gastroduodenal area is believed to occur in approximately 2% or 3% of all patients with Crohn’s disease. The condition can occur without involvement elsewhere in the gastrointestinal tract, but this is extremely uncommon.
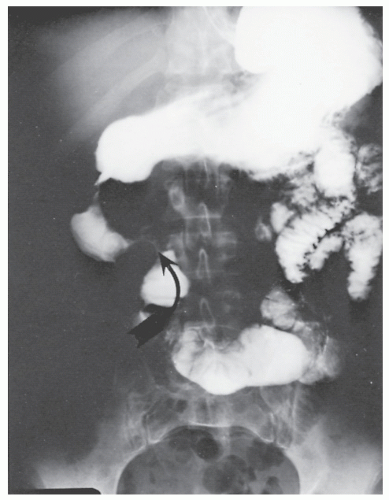
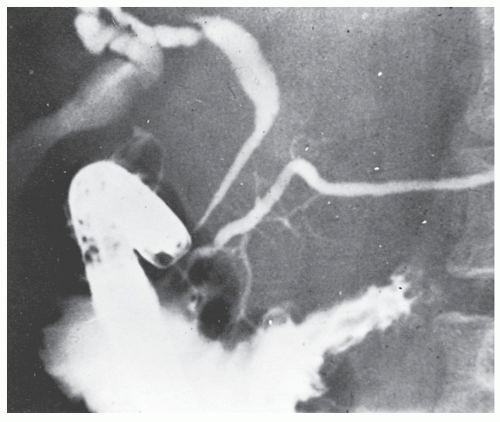
Patients usually present with epigastric abdominal symptoms exacerbated by eating—nausea, vomiting, and weight loss. Symptoms may resemble those of ulcer disease. Obstruction, perforation, fistula, and hemorrhage can occur. A fistula into the stomach characteristically produces symptoms of feculent vomiting, eructation, and odor. Duodenocolic fistula is a recognized complication of duodenal disease, but in evaluation of patients with this finding, it is important to ascertain whether the fistula arose from inflammatory disease of the intestinal tract outside of the duodenum or from the duodenum, itself (see Figures 30-19 and 30-20). Most observers agree that gastroenteric and duodenoenteric fistulas are almost always due to intestinal disease.
Radiologic investigation may reveal the findings summarized by Cohen.91 These include antral inflammation, contiguous disease in the duodenum, cobblestone mucosal appearance with thickened folds, reduced distensibility or stricture, and ulceration (Figure 30-45). Barium enema examination is the preferred study for identifying a fistula between the upper gastrointestinal tract and the colon.
Endoscopic examination may reveal ulceration, cobblestoning, or stricture. As with esophageal disease, the absence of granulomata does not necessarily mean that the patient does not have Crohn’s. Nugent and Roy found granulomas in 37 of 76 individuals (49%).385
Treatment usually consists of antacids and proton pump inhibitors or H2 receptor blockers (an antiulcer program), the medical regimens discussed later, hyperalimentation, and possibly surgical intervention. The primary indications for operation are the presence of a fistula and obstruction (see later). If hemorrhage cannot be controlled by medical means, either resectional surgery or oversewing the bleeding
point is the treatment of choice. Usually, however, surgery for primary gastroduodenal Crohn’s disease can be avoided.
point is the treatment of choice. Usually, however, surgery for primary gastroduodenal Crohn’s disease can be avoided.
Management of Duodenal Stricture
The most commonly performed operative procedure for duodenal Crohn’s disease is gastrojejunostomy, but complications such as bile reflux gastritis, stomal ulceration, blind loop syndrome, and the potential need for a vagotomy are real concerns. Strictureplasty has also been applied for duodenal disease, but in the findings of the Birmingham, England, group, it is associated with a high incidence of postoperative complications, the need for reoperative surgery, and the likelihood of restricture.601 However, our group undertook duodenal strictureplasty on 13 patients and found that it is a safe and effective operation that should be considered when technically feasible.596
A number of cases and reviews have been published on the evaluation and management of the condition as it affects this area.93,100,140,164,255,470,592 Nugent and colleagues reported 18 patients from the Lahey Clinic who were relieved of obstruction by means of gastrojejunostomy.384 This was their preferred treatment for those with this complication. The authors did not advise vagotomy because of the risk of diarrhea and the fact that there was no difference in results between the vagotomized and nonvagotomized groups. A later report from the same institution involved 25 patients who required operation.371 That study revealed that one-third who underwent bypass required reoperation, usually for marginal ulceration or for gastroduodenal obstruction. Although the authors did not feel that the addition of vagotomy protected against the subsequent development of marginal ulceration, they recommend in their latest report that a vagotomy be performed. Shepherd and Alexander-Williams have lent additional support to the concept of vagus nerve interruption when they reported a patient who developed a stomal ulcer 8 weeks following gastroenterostomy without vagotomy.505
Management of Gastric and Duodenal Fistulas
When a fistula develops as a consequence of intestinal disease, simple closure of the stomach or duodenum is all that is usually required, along with resection of the involved bowel segment. Gastric fistulas are always due to disease in the intestine. Treatment of the gastric opening is wedge excision. Occasionally, the opening in the duodenum may occur in an area that is difficult to close, such as adjacent to the pancreas. In this situation and when a large defect is created, an omental or jejunal patch, or the creation of a duodenojejunostomy may be necessary. Lee and Schraut reported one death due to a duodenal leakage in 11 patients with fistulas.300 Greenstein and colleagues noted only nine instances of gastric fistula in a review of 1,480 individuals with Crohn’s disease.200
Results
Ross and associates reviewed the long-term results of surgery for duodenal Crohn’s disease that had been initially reported by Farmer and colleagues at our institution.140,457 Of the 11 patients, 7 required a total of 10 further operations; the mean follow-up was approximately 14 years. Indications for subsequent surgery included marginal ulceration, recurrence producing obstruction at the enteroenterostomy, and duodenal fistula. Eight of the 11 also required surgery for Crohn’s disease elsewhere in the intestinal tract. The authors concluded that bypass surgery alone was unsatisfactory in the long term and suggested that vagotomy be added at the time of operation. Functional results were felt to be better, particularly if reoperative surgery were done in an expeditious and timely manner.
The Lahey Clinic experience now comprises 89 patients.385 Their investigators conclude that irrespective of medical or surgical treatment, duodenal Crohn’s disease follows a more benign course than when it affects the small bowel or colon.
Pancreatic Manifestations
Pancreatitis or pancreatic insufficiency has occasionally been reported with IBD, but this had been felt to be coincidental. One must be aware, however, of the risk of pancreatitis that may be associated with the administration of mercaptopurine (Purinethol). Seyrig and colleagues identified six patients who were thought to have a nonfortuitous association.500 They noted the following, possibly important, clinical distinctions:
Abdominal pain was absent or moderate and probably due to bowel involvement.
Pancreatic calcifications were absent.
Those patients with pancreatic insufficiency had essentially normal pancreatograms.
More information will be necessary before one can establish with certainty whether pancreatic disease is truly an extraintestinal manifestation of IBD.
Hepatobiliary Disease
Liver function studies and liver biopsy often demonstrate abnormal results in both ulcerative colitis and Crohn’s disease patients. Cohen and associates performed a prospective study of liver function in 50 consecutive patients with regional enteritis.90 Thirty percent had abnormal results, most commonly an elevation of the serum alkaline phosphatase, but none had significant liver disease. Fifteen patients of the 19 who underwent liver biopsy had evidence of chronic pericholangitis. Others reported an even higher associated incidence of liver abnormalities.103,118 The reasons for the association between hepatobiliary disease and IBD are not known, but a number of studies have postulated that recurrent cholangitis is due to a portal bacteremia from the interrupted intestinal mucosa, in addition to a probable genetic predisposition.83 Hepatoportal venous gas has been seen in patients with known Crohn’s disease.12
Gallbladder
Cholelithiasis has been reported in up to one-third of patients with IBD, especially in those with Crohn’s ileitis.90 The explanation for this association is believed to involve the enterohepatic circulation. Disease or resection of the terminal ileum leads to loss or malabsorption of bile acids. Because the solubility of cholesterol depends on bile acids, excessive loss may precipitate this substance. This, in turn, may result in stone formation. Another explanation may be the colonization of the terminal ileum by anaerobic bacteria that deconjugate the bile acids to less well-absorbed substances. It is not clear, however, that there is a higher incidence of gallstones in patients with Crohn’s disease than in individuals with ulcerative colitis. Lorusso and colleagues demonstrated an increased risk of gallstones in both conditions, but it was highest in those with Crohn’s disease involving the distal ileum.326 Because of the high prevalence of cholelithiasis in the population, gallbladder imaging has been recommended preoperatively and in the follow-up of IBD patients.290
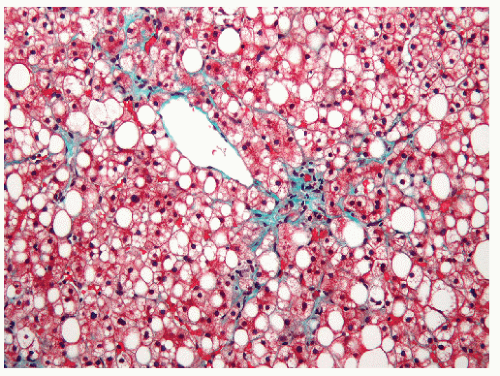 FIGURE 30-46. Fatty degeneration of the liver. Note the fat globules in the liver parenchyma. (Original magnification × 260, Trichrome stain.) |
A different perspective was expressed by Chew and colleagues.82 They retrospectively studied 134 of their patients who had undergone ileocolic resection for Crohn’s disease by means of a questionnaire, using a control group matched for age and gender. There was no significant difference between the groups with respect to prevalence of cholecystectomy. However, those who had more than 30 cm of ileum removed were more likely to have undergone a cholecystectomy. The investigators concluded that synchronous prophylactic cholecystectomy with ileocolic resection cannot be justified based on their data.82
Fatty Degeneration
Fatty degeneration is probably the most frequently encountered microscopic abnormality (Figure 30-46). The incidence has been reported to be as high as 80% and to be due to the relatively poor nutritional state of many colitic patients.83 Occasionally, a granuloma may be seen (see Figure 30-47). Treatment is directed toward correction of the malnutrition.
Pericholangitis
Another common histologic manifestation of liver disease is pericholangitis (Figure 30-47). A more accurate term is portal triaditis because of involvement of bile ductules, portal venules, lymphatics, and hepatic parenchyma.83 The condition may present with jaundice, abdominal pain, fever, and pruritus. Many patients, however, are asymptomatic. Bacterial infection and an autoimmune process have been implicated as possible causative factors. There is no specific treatment for this condition.
Hepatitis
Chronic active hepatitis occurs in only 1% of patients with IBD. Conversely, the incidence of IBD in patients with chronic active hepatitis varies from 4% to 30%.83 Patients have been reported to improve following removal of diseased bowel.
Sclerosing Cholangitis
One of the most serious, albeit rare, consequences of IBD that occurs as a complication of both ulcerative colitis and Crohn’s disease is primary sclerosing cholangitis. Olsson and associates diagnosed this condition in 3.7% of indi-viduals with ulcerative colitis.391 LaRusso and colleagues reported that 70% of their patients with primary sclerosing cholangitis had IBD.295 Broomé and coworkers determined in their evaluation of 76 patients with primary sclerosing cholangitis that histologic changes within the bowel itself may be observed and may actually precede development of clinical symptoms by as much as 7 years.60 The importance of identifying such individuals cannot be overestimated. It has been suggested that even the preclinical manifestations of IBD may subject that individual to an increased risk for the development of malignancy.
Primary sclerosing cholangitis has been much more common in patients with ulcerative colitis than in those with Crohn’s disease. The cause is unknown, but toxins, infectious agents, altered immunity, and a genetic predisposition have been suggested.295 To establish this diagnosis, there must be no prior history of biliary surgery or gallstones, no diffuse involvement of the extrahepatic biliary ducts, and the absence of subsequent development of cholangiocarcinoma.576 Symptoms include right upper quadrant abdominal pain, vomiting, jaundice, and pruritus. Laboratory studies demonstrate the usual changes suggestive of an obstructive jaundice. Cholangiogram reveals a strictured bile duct (Figure 30-48). In contrast to other extraintestinal manifestations of IBD, when the sclerosing cholangitis has been established, removal of the diseased colon does not reverse the condition. The condition is a progressive disease that leads to liver damage and, eventually, liver failure. Liver transplant is the only known cure for primary sclerosing cholangitis, but transplant is typically reserved only for those with severe liver disease. Researchers continue looking into treatments to slow or reverse bile duct damage caused by primary sclerosing cholangitis. But until an effective protocol is found, treatment is directed toward reducing signs and symptoms. Medications and management include a choleretic, such as ursodeoxycholic acid, periodic MR studies of the liver, and as needed endoscopic retrograde cholangiopancreatography (ERCP) with dilation of strictures. Endoscopic dilation of dominant strictures, with
or without stenting, has been shown to alleviate cholestasis and to improve laboratory test results. Monitoring with liver function tests is advisable.
or without stenting, has been shown to alleviate cholestasis and to improve laboratory test results. Monitoring with liver function tests is advisable.
Shaked and coworkers reported their experience with 36 patients who underwent orthotopic liver transplantation for primary sclerosing cholangitis, using immunosuppression with cyclosporine, azathioprine (AZA), and steroids.502 Of these individuals, 29 were known to have chronic ulcerative colitis. The investigators demonstrated that liver replacement and immunosuppression in those suffering from sclerosing cholangitis and ulcerative colitis do not alter the course of the colonic disease. Bleday and colleagues have demonstrated that there appears to be a group of patients who have undergone liver transplant who rapidly develop colorectal malignancy.49 These individuals require frequent, long-term surveillance following transplant. This suggests that the immunosuppressive agents employed for managing patients who have undergone orthotopic liver transplantation may have a pejorative effect on the colon through increased predisposition for the development of malignancy. But there is no evidence to suggest an association between sclerosing cholangitis itself and colorectal carcinoma in patients with IBD.382 However, a number of cases of carcinoma of the gallbladder have been described in individuals with sclerosing cholangitis and ulcerative colitis.119
Interestingly, despite massive immunosuppression associated with transplanting small intestine, histologically confirmed recurrent Crohn’s disease has been demonstrated in the transplanted bowel.539
Cangemi and colleagues prospectively compared the progression of clinical, biochemical, cholangiographic, and hepatic histologic features in 45 patients with both primary sclerosing cholangitis and ulcerative colitis, 20 of whom underwent proctocolectomy and 25 of whom had not.72 No beneficial effect was seen as a consequence of the operation. Because of the profoundly serious consequences of progressive cholangitis, a case may be made for “prophylactic” removal of the inflammatory bowel process if early changes in the biliary tract are observed. This has been suggested even when the gastrointestinal manifestations are quite minimal, but there is no evidence to support implementation of this concept. Still, if surgical treatment is needed for the IBD itself, those with well-controlled primary sclerosing cholangitis can undergo such operations as restorative proctocolectomy safely (for ulcerative colitis).415
Cirrhosis
Although cirrhosis is an uncommon complication of IBD, it has, in the past at least, been felt to cause 10% of deaths.83 When it occurs it is usually a consequence of sclerosing cholangitis. Patients may develop the characteristic stigmata of portal hypertension, including bleeding esophageal varices, and ileostomy hemorrhage (see Chapter 31).
Carcinoma of the Bile Duct
Carcinoma of the bile duct arising in a patient with ulcerative colitis is a rare complication. The association was originally described by Parker and Kendall in 1954.402 In 1974, Ritchie and colleagues identified 67 cases.450 The condition is more common in men and is usually seen in patients who have had a prolonged history of colitis. Patients give a history of typical biliary obstruction with painless jaundice, weight loss, and pruritic symptoms. Diagnosis is usually confirmed by ultrasound demonstration of dilated intrahepatic ducts and by endoscopic retrograde cholangiography. Prognosis is poor, with biliary diversion the usual surgical approach.587
Cutaneous Manifestations
Pyoderma Gangrenosum
Pyoderma gangrenosum is a condition found exclusively in individuals with IBD but is fortunately uncommon,
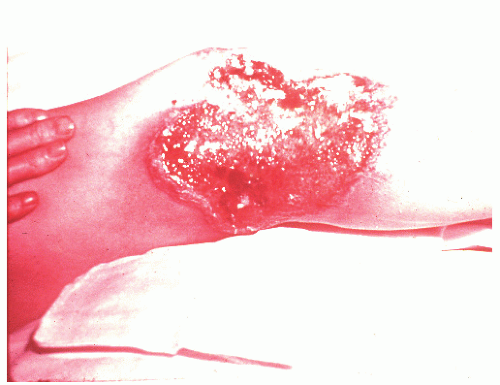
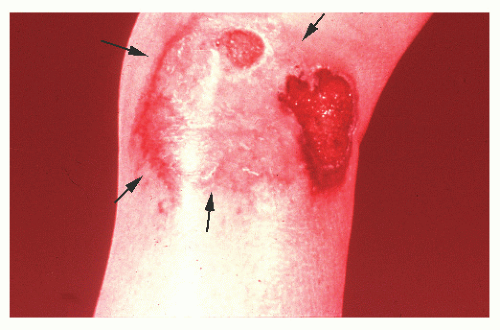
occurring in no more than 2% of patients.362 Schoetz and associates identified 8 of 961 with Crohn’s disease (an incidence of 0.8%).495 The vast majority of patients have active intestinal disease at the time the pyoderma develops, although in rare cases the skin lesions may antedate apparent bowel involvement.362 Clinically, the lesion appears as a spreading, undermining ulceration that has a characteristic violaceous border (Figures 30-49 and 30-50). It is usually found on the extremities, the most common location being the anterior tibial area.419 However, the ulcers can occur on the trunk, buttocks, and other places. Usually, there are only one or two lesions, but these can be of considerable size.
occurring in no more than 2% of patients.362 Schoetz and associates identified 8 of 961 with Crohn’s disease (an incidence of 0.8%).495 The vast majority of patients have active intestinal disease at the time the pyoderma develops, although in rare cases the skin lesions may antedate apparent bowel involvement.362 Clinically, the lesion appears as a spreading, undermining ulceration that has a characteristic violaceous border (Figures 30-49 and 30-50). It is usually found on the extremities, the most common location being the anterior tibial area.419 However, the ulcers can occur on the trunk, buttocks, and other places. Usually, there are only one or two lesions, but these can be of considerable size.
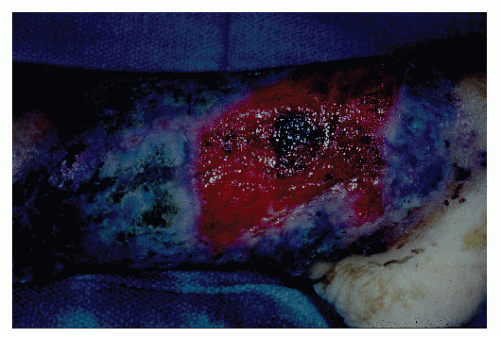 FIGURE 30-49. Pyoderma gangrenosum. Irregularly outlined, sharply defined ulceration with edematous edges and pyodermatous base in a patient with ulcerative colitis. (Courtesy of Rudolf Garret, MD.) |
Biopsy shows no definite characteristics that would identify the ulcer as being specific for a complication associated with IBD. A vasculitis has been suggested as a possible etiology.
Treatment consists of administration of systemic steroids and occasionally intralesional steroids, and, of course, the management of the colitis.186 Successful response to topical disodium cromoglycate (DSG) has been reported.77 Because it is known that DSG prevents the release of histamine from mast cells, an allergic component may be involved in the mechanism for its efficacy. Topical measures also should include appropriate antibiotics if culture suggests the value of such treatment or if lymphangitis or cellulitis is present. As with so many other extraintestinal manifestations, the course of the pyoderma parallels the clinical progress of the intestinal disease.
Rarely does the skin condition assume such significance that colectomy must be performed for this indication alone. A total colectomy with preservation of the rectum may result in incomplete healing of the pyoderma, but the residual skin problem can fail to clear until proctectomy is subsequently performed (Figure 30-51).
A variant of this manifestation is parastomal gangrenosum, a paraileostomy ulceration/fistula. In this condition, there are multiple cutaneous defects surrounding the stoma. These may drain pus and are exquisitely painful. One can probe the external openings, but there is no evident fistulous communication. These are characteristically subcutaneous and may lead to overlying skin necrosis. There is usually a communication at the mucocutaneous attachment of the stoma. This is different from that of pyoderma in that this condition usually appears away from the mucocutaneous junction.
Treatment of parastomal gangrenosum involves unroofing of these extensions. In severe cases, this may lead to circumferential excision of skin, leaving a defect that may make pouch security impossible. The defect in the subcutaneous tissue harbors exuberant granulation tissue, which is curetted down to the base of the ulcer. Pouching is accomplished by using a nonseal appliance. Application of layered cotton dressings soaked in Domeboro’s solution is used over the defect. A Perry model no. 51 sleeve is then applied to the ostomy (see Chapter 32). This is kept in place with straps applied to belt hooks. The dressing is changed daily. Defect closure by secondary intention may be accomplished in 4 to 6 weeks. For lesser degrees of parastomal ulceration, a Telfa dressing is applied. The stoma is then pouched with daily changes. Stomal relocation is rarely required.
Polyarteritis Nodosa
Polyarteritis nodosa is a rare cutaneous manifestation of Crohn’s disease. Kahn and colleagues reviewed 11 cases in the literature and added one of their own.316 The presence of erythematous, tender nodules in the extremities should lead one to suspect the diagnosis. Biopsy or excision may reveal an arteritis with luminal narrowing by fibrinous thrombus.
The relationship of the cutaneous manifestation to systemic polyarteritis nodosa is controversial, but in the case reported by the authors, when subsequent resection of the bowel was carried out, there was no evidence of such an arteritis. The condition should be distinguished from other cutaneous manifestations, such as those in the following discussion.
Erythema Nodosum
Erythema nodosum is another cutaneous manifestation that is relatively uncommonly seen with IBD with up to 5% of patients reported to be afflicted.259 In a report from our institution, 90% had active bowel disease at the time skin lesions developed.362 In their experience, erythema nodosum typically occurred as a single episode and lasted for several days, associated with active bowel disease and joint symptoms. Tender, subcutaneous nodules are usually seen on the pretibial aspects of the legs. As with other extracolonic manifestations, the clinical course usually parallels that of the intestinal disease.
Psoriasis
There appears to be an increased risk for the development of psoriasis in patients with IBD.250,454 In a report by Yates and colleagues, the prevalence of psoriasis in Crohn’s disease (11.2%) and in ulcerative colitis (5.7%) was significantly greater than that of the control group (1.5%).607 The increased association of the two conditions as well as the higher rate of psoriasis in first-degree relatives of Crohn’s patients implies the possibility of a genetic link.299
Cutaneous Crohn’s Disease
Crohn’s disease of the skin can develop with the characteristic histologic feature of the bowel condition—specifically, granulomatous inflammation of the dermis. When this occurs other than by direct extension from the gastrointestinal tract, it has been given what one may consider to be a poor but now accepted term, metastatic cutaneous Crohn’s disease. Fewer than 50 cases have been reported.210,311,516,541,564 The condition has a variable macroscopic appearance, including ulceration, erythema, and nodularity. Biopsy is required to establish the diagnosis, with differentiation from sarcoidosis a potential problem, because the two diseases have similar cutaneous findings.350 Treatment by means of intralesional steroid therapy has been attempted with usually transient improvement. Therapeutic benefit has also been achieved with oral metronidazole (Flagyl).52,496
Arthritis and Rheumatologic Conditions
Depending on the interpretation of what truly constitutes arthritic or rheumatologic conditions associated with IBD, there are perhaps as many as four clinical patterns: first, a peripheral joint synovitis that is closely related to the activity of the bowel disease (15% to 20% of patients); second, ankylosing spondylitis (3% to 6%), in which the relationship with the bowel disorder is less clearly defined; third, a bilateral symmetric sacroiliitis (5% to 15%); and a fourth category, which includes rheumatic complications, such as granulomas of bones and joints, clubbing, periostitis, osteomalacia, osteoporosis, septic arthritis, and complications of corticosteroid therapy.193,378
Colitic Arthritis
Colitic arthritis or enteropathic arthritis is the most common joint manifestation of IBD and is seen more frequently with Crohn’s disease than with ulcerative colitis. The large joints are primarily involved (knees, ankles, elbows, and wrists). They may be swollen, warm, and red (Figure 30-52). The appearance may not be dissimilar to that of rheumatoid arthritis, but it is nondeforming and seronegative: that is, rheumatoid factor is absent from the blood. Although any joint may be attacked, small joints are less frequently affected (Figure 30-53).
Symptoms of the arthritis usually develop after the IBD has been diagnosed and tend to parallel the course of the intestinal disease. The inflammation is usually adequately controlled by means of anti-inflammatory agents or by the use of steroids. The arthritis completely resolves after colectomy.
Rheumatoid Spondylitis
As stated, other joint disorders that may be encountered are severe arthralgias and rheumatoid spondylitis.1,193,256,348,613 The incidence of rheumatoid spondylitis is considerably higher in patients with IBD than in the general population, with estimates ranging in excess of 20 times. The wellknown gender incidence (4:1 ratio of men to women) is reversed when rheumatoid spondylitis complicates IBD. A genetic association between the two diseases has been demonstrated. In contradistinction to most other extracolonic manifestations, spondylitis does not parallel the activity of the bowel disease. The incidence is similar in Crohn’s disease and ulcerative colitis.
The patient initially develops pain in the lumbosacral region, but the discomfort may rapidly progress to involve the thoracic and cervical spine. As ankylosis progresses, the patient exhibits the characteristic dorsal kyphosis (Figure 30-54). Interestingly, isolated asymptomatic sacroiliitis occurs more often than does spinal involvement (4% to 18%).193
Treatment consists of physiotherapy, anti-inflammatory agents, and steroids. Colectomy is not indicated for the treatment of the arthritic manifestations.
Hypertrophic Osteoarthropathy or Finger Clubbing
Finger clubbing has also been reported in association with IBD. It may regress after resection of the involved bowel segment and usually correlates with disease activity.161
Polymyositis
Polymyositis has been reported on rare occasions to be a condition associated with IBD, especially ulcerative colitis.84 As with other unusual extraintestinal manifestations, one must always be concerned about the possibility that the condition may be simply coincidental. Still, because of the possible autoimmune etiology of the two diseases, a causal link may be considered plausible.
Bronchopulmonary Disease
Pulmonary manifestations have been said to be associated with IBD, including bronchiectasis, granulomatous lung disease, interstitial fibrosis, and sulfasalazine pneumonitis.14,305 Storch and coworkers reported more than 400 instances, categorizing the cases by disease mechanism into drug-induced disease, anatomic disease, overlap syndromes, autoimmune disease, physiologic consequences of IBD, pulmonary function test abnormalities, and nonspecific lung disease.537 The authors conclude that manifestations of IBD in the lung vary and often present a confounding diagnostic problem, necessitating a complex workup.537 Investigation of respiratory factors in patients with Crohn’s disease free of clinical pulmonary symptoms and with normal chest roentgenograms was studied by Bonniere and colleagues.54 This included serum angiotensin-converting enzyme, pulmonary function tests, bronchoalveolar lavage, and pulmonary scanning. Results suggest that most patients with Crohn’s disease have a much higher frequency of latent lung abnormalities than would be expected in a control population.
Ocular Manifestations
Ocular disease including orbital congestion, uveitis, conjunctivitis, iritis, and keratitis are found in up to 10% of patients with IBD. The most common ocular lesion is episcleritis, an inflammation overlying the sclera, under the conjunctiva. A thickened, deep red appearance is usually noted in one segment of the eye (Figure 30-55). Burning, itching, and pain are the primary symptoms, but the patient often seeks medical attention because of the appearance. Steroids and anti-inflammatory agents are the treatments of choice. Episcleritis is often associated with exacerbations of the underlying IBD but is unrelated to extent or severity.14 Because of the complications that may lead to a chorioretinitis, ophthalmologic consultation is advised.
Uveitis usually produces ocular pain, blurred vision, and headache and may occur whether the underlying disease is symptomatic or in remission.14 The diagnosis is established by slit lamp examination. Treatment consists of pupillary dilatation to relieve spasm, an eye patch, and the application of topical corticosteroids.
Amyloidosis
Secondary amyloidosis was initially described in 1949 by Cohen and Fishman in a patient with IBD and is still a rare reported finding.88 When recognized, it occurs almost exclusively with Crohn’s disease. The diagnosis has been made usually at postmortem examination, although confirmation has been established in a few patients who presented with renal failure and who underwent renal biopsy.14 In the absence of kidney dysfunction, a search for secondary amyloidosis is probably not justified.327 The application of rectal biopsy for establishing the diagnosis is discussed in Chapter 26. Regression of proteinuria and other manifestations of renal as well as hepatic dysfunction has been reported following bowel resection.128,163,181
Urologic Complications
Urologic complications are commonly seen in association with IBD. These include chronic interstitial nephritis, chronic pyelonephritis, acute tubular necrosis, urinary fistulas, ureteral obstruction, and nephrolithiasis.
Ureteral Obstruction
Ureteral obstruction is much more frequently identified in association with Crohn’s disease than with ulcerative colitis (Figure 30-56). This is due to periureteric fibrosis. The incidence has been reported to be as high as 50% in this group of patients. An inflammatory mass involving the terminal ileum and occasionally the sigmoid colon can produce extrinsic compression of the distal ureters, with the right more frequently involved than the left. The result is hydroureter, hydronephrosis, and possibly, caliectasis. Symptoms related to the urinary tract may be minimal and, if present, may actually be due to compression of the bladder by an inflammatory mass rather than to the obstruction of a ureter. Flank pain is suggestive of ureteral obstruction.
Treatment involves removal of the mass that is causing the compression. In the past, ureterolysis had been advocated, but this risks ureteric ischemia. Complete resolution may be expected, assuming that the obstruction has not produced irreversible renal damage. Indeed, in some cases, drainage of the accompanying abscess alone will be sufficient to relieve the obstruction. Preoperative identification of ureteral dilatation should warn the surgeon that a meticulous dissection may be required to avoid ureteral injury (see Chapter 24). The preoperative placement of ureteral catheters should be considered.
Nephrolithiasis and Bladder Calculi
Nephrolithiasis and bladder calculi are seen in at least 5% of individuals with IBD. Following proctocolectomy and ileostomy, patients are still at risk for the development of nephrolithiasis. Many factors contribute to the development of calculi in chronic IBD: decreased urine volume, increased crystalloid concentration, urinary electrolyte and pH changes, and recurrent urinary tract infections. The more acid urine favors precipitation of urates, and, as a result, uric acid calculi are more common in patients with IBD than in the “normal” population of stone formers. Intestinal absorption of oxylate is increased when ileal disease is present, and this is a further reason for formation of stones. Figure 30-57 demonstrates numerous bladder calculi that developed in a 60-year-old man who had extensive Crohn’s disease of the small intestine for more than 20 years.
Bambach and associates reported that 10% of patients who underwent resection for IBD gave a history of urinary stone formation after surgery.29 Ileostomy patients were demonstrated to have a significantly lowered urinary pH and volume and a higher concentration of calcium, oxylate, and uric acid. The authors advise close follow-up of patients with ileostomies and with small bowel resections in particular, in order to assess fecal losses and urinary composition. Ideally, patients who are at an increased risk for the formation of urinary stones could then be identified.
An adequate urinary volume should be maintained by encouraging the patient to consume sufficient water to increase urinary volume without increasing ileostomy output, by alkalinization in selected patients, by a diet low in oxylate and fat, and by “slowing” medications to reduce the ileostomy volume.29
Psoas Abscess
Psoas abscess has long been recognized as a complication of Crohn’s disease, but it has also been found, albeit rarely, with ulcerative colitis.4,572 The condition is secondary to posterior perforation of the diseased organ. In our experience, 73% of individuals presenting with this manifestation had regional enteritis.431 When seen as a consequence of Crohn’s disease, it may be the result of a fistula or abscess from the terminal ileum on the right side or of disease of the jejunum or sigmoid on the left.308 Pain in the iliac fossa, abdomen,
groin, or hip is the usual symptom. Chills and fever, abdominal or flank mass, and an associated cutaneous fistula may be apparent.
groin, or hip is the usual symptom. Chills and fever, abdominal or flank mass, and an associated cutaneous fistula may be apparent.
In the past, a limited open drainage (a so-called finger laparotomy) was done. Currently, CT-guided abscess drainage is favored. After sepsis has resolved, usually within 6 weeks, then laparotomy and resection of the source—namely, ileocolic resection or, in certain cases, sigmoid resection— may be done.
Operating on a psoas abscess may require an extensive dissection. There may even be a horseshoe appearance with extension up one or both retroperitoneal gutters. Curettage of the retroperitoneal tract is carried out with great care. For tracts extending into the deep pelvis, curettage may provoke alarming hemorrhage from disruption of pelvic branches of the iliac vessels. The tract is then drained through a lateral incision. Following resection of the diseased bowel segment, omentum is placed between the curetted abscess tract and the anastomosis in order to minimize the risk of reperforation. Extension of the septic process with establishment of a spinal extradural abscess requiring emergency laminectomy has been reported.6
Cardiac Complications
Cardiac complications in association with IBD are extremely unusual, with pericarditis being the most frequently described. Ballinger and Farthing postulate that heart block may, in some individuals, be a complication of ulcerative colitis and not a coincidental association.28 Others opine that IBD may be considered an independent risk factor for the development of bacterial endocarditis.288 Therefore, endocarditis prophylaxis should be considered, according to some investigators, even in the absence of predisposing primary cardiac factors for the development of bacterial endocarditis.288
Other Manifestations
A number of other conditions have been suggested to be associated with nonspecific IBD. Evidence of Crohn’s disease has been found in voluntary muscle, the larynx, and the ovary. Whether these and other manifestations such as hyperthyroidism, hyperparathyroidism, and hematologic problems are coincidental has not been clearly established.
Relationship to Carcinoma
For many years, it was felt that there was no increased risk for the development of carcinoma in patients who had Crohn’s disease. However, an accumulation of published reports beginning in the 1960s seems to indicate an exponential increase in the frequency of this observation.461 Some have suggested that the observations are merely coincidental. Most investigators, however, accept the thesis that such a relationship does indeed exist.15,41,57,94,178,195,214,284,293,444,486,529,606 This association is not equivalent to that which has been observed in long-standing, chronic ulcerative colitis, however.
In 1980, Zinkin and Brandwein identified 43 patients with adenocarcinoma arising in a segment of Crohn’s colitis and added one of their own.612 Hawker and associates reviewed the literature on Crohn’s disease of the small intestine and identified 61 cases, including their own experience.230 There
were 41 tumors of the ileum, 18 of the jejunum, 1 in the duodenum and ileum, and 1 in the ileum and colon. Eighteen occurred in bypassed intestinal loops. More recently, Rubio and colleagues found 174 small and large bowel cancers occurring in Crohn’s disease in their literature review. Stahl and coworkers identified 22 patients with malignancies at the Lahey Clinic.529 The median age at diagnosis of Crohn’s disease was 37 years and at diagnosis of carcinoma was 54 years.
were 41 tumors of the ileum, 18 of the jejunum, 1 in the duodenum and ileum, and 1 in the ileum and colon. Eighteen occurred in bypassed intestinal loops. More recently, Rubio and colleagues found 174 small and large bowel cancers occurring in Crohn’s disease in their literature review. Stahl and coworkers identified 22 patients with malignancies at the Lahey Clinic.529 The median age at diagnosis of Crohn’s disease was 37 years and at diagnosis of carcinoma was 54 years.
It is very difficult to assess the true incidence of Crohn’s disease and carcinoma in the small intestine because small bowel carcinoma is such a rare condition. However, the association appears genuine and is based on several observations. There is a different distribution of small bowel cancer in Crohn’s disease when compared with cancer of the small intestine that occurs in the absence of this inflammatory condition. In Crohn’s disease, two-thirds of the small bowel cancers occur in the ileum, whereas no more than 30% develop in this area when it is not involved by disease.230 In the large intestine, Crohn’s disease with cancer is usually found on the right side of the colon, as compared with the usual more distal bowel involvement in the general population. In a study from Birmingham, England, Gyde and colleagues demonstrated a significant excess of tumors in both the upper and lower gastrointestinal tract in patients with Crohn’s disease.213 They further demonstrated that the whole tract may be at an increased risk and that this risk is not confined to areas of obvious inflammatory involvement. Additional features of carcinoma in Crohn’s disease include multifocal lesions and metachronous intestinal and extraintestinal cancers. Patients who develop carcinoma in Crohn’s disease are usually relatively young, and more than one-half with colonic cancer are younger than the age of 40 years.520 A long duration of illness also appears to be an underlying factor, and there is perhaps a tenfold greater risk of developing colon cancer in this circumstance than in the general population.447
The likelihood of the development of carcinoma is greatly enhanced in patients who have undergone intestinal bypass.202,214,230 This is particularly true when chronic inflammatory disease persists for many years. Lavery and Jagelman identified two cases of carcinoma that developed in the out-of-circuit rectum after subtotal colectomy and ileostomy for Crohn’s disease.296 The mortality rate from patients who develop cancer in excluded bowel is extremely high (>80%), probably because recognition is very late in the progression of the disease.195 Even without an exclusion or bypass procedure having been performed, survival is poor with small bowel cancer. In the experience of Michelassi and coworkers, the mean survival rate was only 6 months compared with 65 months for those with large bowel cancer.359 The observation of new signs and symptoms after a prolonged period of quiescence, particularly with long-standing disease, and especially if the patient had previously undergone an exclusion or bypass procedure, should be vigorously evaluated for the possible presence of a malignancy.
This change in symptoms after a period of quiescence is of critical concern also with respect to prior strictureplasty (see later). Ribeiro and colleagues recommend that all strictures be widely opened and carefully examined prior to strictureplasty, with frozen-section biopsies of all suspicious areas.444 Although the risk of adenocarcinoma developing at the site of a strictureplasty is quite remote, several such instances have been reported.257,336
Anal Crohn’s disease also appears to be associated with an increased risk for the development of anal carcinoma.57,293,517,524 Malignant changes may be inapparent, especially when the tumor arises in a fistula. Pain, stricture, and induration often preclude the performance of an adequate examination.293 Deterioration of an individual’s perianal symptoms and clinical findings warrants investigation and possible biopsy.57 There also seems to be a relationship with intestinal-cutaneous fistulas, although there is always the question of whether the fistula may be secondary to an underlying malignancy in the intestine.86 Whether the anal or abdominal fistula causes malignant change to occur by “irritation” or by a stimulus to mucosal regeneration is a matter for conjecture.
Although carcinoma arising at the stomal site in patients with polyposis or ulcerative colitis has been described (see Chapter 31), it was only recently that the complication has been reported in Crohn’s disease.507 In both of the cases, dysplasia was identified in adjacent tissue.
Specific symptoms suggestive of malignancy are rarely identified. However, the important issue, as stated, is that any change, especially following a period of quiescence, demands investigation. Cancer should also be considered when complete obstruction fails to resolve with adequate decompression.444
Dysplasia
As with carcinoma in ulcerative colitis, radiologic diagnosis of malignant change in patients with Crohn’s disease is virtually impossible. Endoscopic examination likewise is of very little benefit in establishing the diagnosis. However, dysplasia, if present, probably is as significant a finding as it is with ulcerative colitis.185,214,408 The dysplasia that is recognized is essentially identical to that described in patients with ulcerative colitis (see Chapter 29).179,279,445,447,460,461 Unfortunately, with the exception of the very distal ileum, the small intestine does not lend itself to investigation by means of biopsy, especially if the segment is excluded. It is probably a reasonable precaution to recall all patients who have undergone a bypass or an exclusion procedure and evaluate them for resection.
Korelitz and colleagues analyzed 356 patients with Crohn’s disease by means of rectal biopsy in order to study epithelial dysplasia.279 Eighteen (5%) exhibited this finding, including those with a normal-appearing mucosa. Colorectal carcinoma was found in 11% of these individuals. Four patients developed carcinoma who did not have dysplasia on rectal biopsy. Löfberg and colleagues evaluated 24 patients with long-standing colonic Crohn’s disease by means of colonoscopic biopsy at 10 predetermined sites.322 Although none had definite dysplasia, three demonstrated DNA aneuploidy, one of whom subsequently developed a carcinoma. The authors believe that dysplasia is rare in Crohn’s disease but that DNA aneuploidy needs to be carefully assessed. Obviously, further studies are needed. Richards and associates suggest that surveillance of the colon should commence after 10 years of disease.447 How frequently this should be undertaken is still not determined, but the issues and concerns expressed in Chapter 29 should be well heeded.
Comment
As was discussed in Chapter 29, the concept of surveillance to identify dysplasia before malignancy supervenes has been subjected to a more critical analysis in recent years. The
same should be true for the concept in the management of patients with Crohn’s disease. A statement made by Sachar is worth quoting completely because it reflects my own views on this subject.
same should be true for the concept in the management of patients with Crohn’s disease. A statement made by Sachar is worth quoting completely because it reflects my own views on this subject.
The most rationale position, therefore, in view of the equivalent risks, might be to adopt equivalent policies for the two diseases. In other words, debate the pros and cons of surveillance programs to your heart’s content; but in the end, whatever you choose to do for your patients with ulcerative colitis, do no differently for those with Crohn’s colitis of similar duration and extent.473
Other Malignancies
The issue of extraintestinal malignancies with IBD is a matter of some controversy. Whether the association is valid or merely coincidental is still unresolved. Concomitant malignant melanoma and lymphoma have been suggested to be more than mere coincidence, perhaps related to immunosuppression from the disease itself or from medical treatment.148,152,199,203
Medical Management
The reader is directed to Chapter 29 for a comprehensive discussion of the medical management of ulcerative colitis.
The management of Crohn’s disease is dependent on the assessment of the disease location, the severity, the exclusion of abscess, and the elimination of extraluminal factors, such as cigarette smoking, nonsteroidal anti-inflammatory drugs, Clostridium difficile, acute self-limited infections, or irritable bowel-like symptoms that might better respond to conventional therapy.220 Confounding factors, of course, include undetected but coexistent carcinoma and AIDS. Dietary problems referable to sorbitol or lactose may obscure the initial concern that this is actually Crohn’s disease. Approaches to the management of patients include lifestyle, diet, and pharmaceuticals, each of which may independently aid in the management of the asymptomatic individual with Crohn’s disease.241 The therapeutic goals are to induce a clinical remission, to maintain that remission, and to prevent postoperative relapse.
The plethora of information regarding pathogenetic mechanisms in the understanding of Crohn’s disease has led to a number of imaginative and innovative treatment approaches. Kornbluth and colleagues undertook a study to determine the efficacy of various medical therapies in the treatment of severe IBD based on a MEDLINE computer-assisted literature search.283 They determined that clinical remission was achieved on average in 65% of individuals with Crohn’s disease. They concluded that current medical therapy for severe Crohn’s colitis seems to spare many patients early colectomy. The Crohn’s Disease Activity Index (CDAI), which consists of the clinical variables correlated with the physician’s assessment of the patient’s well-being, although validated, has been criticized because of its subjectivity and interobserver variability.218
Mild-to-Moderate Disease
In mild-to-moderate disease, the initial drug of choice is 5-ASA in a form targeted to the inflammatory site, using higher doses than that which is required for ulcerative colitis. It is anticipated that there will be a 40% to 60% response rate.515 Metronidazole and ciprofloxacin regimens can produce results similar to those of 5-ASA, particularly in Crohn’s ileocolitis and in perianal disease.421,540 However, these observations have been challenged for induction of remission with either high-dose 5-ASA or antibiotics and certainly are not as impressive as those with immunomodulator therapy.222
Moderate-to-Severe Disease
Moderate-to-severe disease almost always requires steroids initially, but only for short-term use. No long-term benefit has been proven. However, there is an 80% relapse rate when steroids are terminated prematurely. The concern, of course, is that 35% of patients may become steroid-dependent in 1 year, but 20% remain in remission.369 There is no value in low-dose steroids in preventing relapse.
Severe Disease
In severe Crohn’s disease, patients are admitted into the hospital and treated with intravenous medications only (Figure 30-58). There have been no unequivocal data that intravenous cyclosporine has a therapeutic advantage over intravenous corticosteroids, nor has a sustained benefit been demonstrated in Crohn’s disease.153 This has been particularly true with the low dose and the oral route. Drainage of abscess is, of course, mandatory.
Maintenance of Remission
In maintaining remission, the maintenance dose of 5-ASA is the inductive dose—that is, the dose that prevents relapse or recurrence after surgery. However, it may be less effective if steroids, 6-mercaptopurine (6-MP), or AZA induced that remission. If steroids did induce the remission, there is a better
chance of maintaining that remission with 6-MP and AZA (or possibly methotrexate) than with continued use of 5-ASA.220
chance of maintaining that remission with 6-MP and AZA (or possibly methotrexate) than with continued use of 5-ASA.220
Postoperative Recurrence
The maintenance of remission following resection for Crohn’s disease continues to be a topic of concern among internists, gastroenterologists, and surgeons. Symptomatic relapses occur at a rate of 40% to 70% in most series within 2 years following operation.175 Endoscopic recurrence actually increases that rate to 80%. In the natural course of the disease, the inflammatory process may tend to extend distally, but rarely proximally. However, once the patient undergoes surgery, proximal involvement is more likely to develop, usually at and just above the anastomosis.
Korelitz believed that sulfasalazine is more effective in preventing recurrence in Crohn’s disease than it is with ulcerative colitis.275 However, the initial enthusiasm for the value of 5-ASA in reducing the likelihood of postoperative recurrence has not been supported by later investigations.59 The European Cooperative Crohn’s Disease Study showed no significant reduction in the clinical relapse rate.318 Yet a meta-analysis of the 5-ASA experience did show an effect on reducing recurrence postoperatively.2
There is, however, value to the postoperative use of immunomodulators and some short-term improvement with metronidazole if side effects can be tolerated.278 Further study of 55 postoperative Crohn’s patients randomized to 6-MP or control showed no difference in time to relapse, but the postoperative endoscopic score of disease activity was significantly lower in the 6-MP patients.48
Aminosalicylates
The primary drugs that have been traditionally employed for the treatment of Crohn’s disease are sulfasalazine and steroids (see Chapter 29). Aminosalicylates can delay recurrence, but concern has been expressed about the clinical efficacy of 5-ASA and antibiotics in Crohn’s disease.222 For example, sulfasalazine has been shown to have a minimal effect on inducing remission in active Crohn’s disease when compared with a placebo.538 Moreover, convincing evidence is lacking that mesalamine is effective for treatment of active Crohn’s disease. Gendre and colleagues employed a placebo-controlled study with the use of oral mesalamine (Pentasa) for maintenance therapy and found a statistically significant difference in the maintenance of quiescence in the treated group.175 Likewise, Brignola and coworkers performed a controlled study with mesalamine and found a statistically significant difference in the incidence of endoscopic lesions and severity in the treated group compared with the placebo.59 A similar favorable result was observed in individuals who were treated with oral 5-ASA (Asacol).422
Sandborn and Feagan suggest an alternative treatment algorithm as a result of their analysis of randomized controlled trials for mild-to-moderate Crohn’s disease.480 Their evidence-based conclusions are as follows:
Colonic disease.
Sulfasalazine for colonic Crohn’s induction of remission with budesonide 9 mg/day for ileal or right disease.
Conventional steroids for more severe disease activity, budesonide failures, and patients allergic to or intolerant of sulfasalazine.
Mesalamine has limited value in preventing relapse after a medically induced remission, especially if it has been steroid-induced, or in maintaining remission postoperatively.69,318 The question of mesalamine’s role in preventing postoperative recurrence of Crohn’s disease has been revisited by Caprilli and coworkers who found no clinically significant advantage between 84 patients receiving 4.0 g/day (12%) versus 81 patients given 2.4 g/day (14%).74
Steroids
A dramatic response is often observed when intravenous steroids are administered. A mass may rapidly disappear, or a patient’s acute abdominal symptoms may resolve. The natural history of corticosteroid use in IBD was reviewed in 173 patients treated in Olmstead County, Minnesota, a communitybased experience rather than a study of patients referred to a tertiary medical center.146 Of 74 individuals (43%) treated with corticosteroids, 43 (58%) entered complete remission, 19 (26%) had a partial remission, and 12 (16%) had no response. At 1 year, however, only 24 (32%) still had a prolonged response, yet 21 (28%) were steroid dependent, and 38 required surgery (38%). Thus, of 84% initial responders, only 32% maintained that response free of steroids and without surgery. It should be noted that only 38% of Crohn’s patients in this population needed steroids, thus speaking for mild disease. A second lesson learned was that the need for steroids in this group suggested a poorer prognosis and a higher likelihood that surgery would be required.
A double-blind study of the effectiveness of sulfasalazine compared with 6-methylprednisolone in patients with Crohn’s disease revealed that the addition of the former drug offered no advantage.333
Budesonide (Entocort) in ileocolonic Crohn’s disease produced a 45% improvement in 8 weeks in the Canadian experience, with no significant long-term steroid side effects, and with a remission rate at 8 weeks comparable to that of prednisone, with less adrenal suppression.71 Both prednisone and budesonide induced remission in approximately 60% of patients, with fewer side effects with budesonide in the Italian and Israeli experience.31,70,395,469 Short-term use of budesonide appeared superior to that of mesalamine (5-ASA) in preventing relapse at 1 year in steroid-dependent patients who were intolerant to AZA/6-MP.176
Budesonide may be a reasonable alternative as replacement therapy in glucocorticoid-dependent patients. Cortot and colleagues described 118 individuals with inactive Crohn’s disease who were randomized to budesonide, 6 mg, or placebo, while tapering prednisolone.95 At 13 weeks, 68% of budesonide patients were in remission versus 35% of placebo patients. There was a demonstrable reduction of glucocorticoid side effects—that is, moon face, acne, insomnia, and mood swings—in both the budesonide and the placebo groups.
In a meta-analysis, budesonide was less likely to induce remission in active Crohn’s disease than conventional corticosteroids but was better than mesalamine or placebo over 8 weeks and with fewer steroid adverse events.396 There appears to be a role for budesonide as pulse therapy for quickly inducing a remission for a Crohn’s flare and using it as bridge therapy until AZA/6-MP can “kick in.”
Antibiotics (Metronidazole [Flagyl] and Ciprofloxacin [Cipro])
Metronidazole was initially introduced for the treatment of Trichomonas vaginalis infections but was subsequently
demonstrated to be a very effective antibiotic against anaerobic organisms. It was also found to have activity against gram-positive and gram-negative bacteria. The drug is a substituted imidazole that is rapidly absorbed orally and rectally and can be given intravenously to the acutely ill patient. A number of theories suggesting the mechanism of its action have been proposed: immunosuppression, an effect on wound healing, stimulation of leukocyte chemotaxis, and, of course, its antimicrobial effect.
demonstrated to be a very effective antibiotic against anaerobic organisms. It was also found to have activity against gram-positive and gram-negative bacteria. The drug is a substituted imidazole that is rapidly absorbed orally and rectally and can be given intravenously to the acutely ill patient. A number of theories suggesting the mechanism of its action have been proposed: immunosuppression, an effect on wound healing, stimulation of leukocyte chemotaxis, and, of course, its antimicrobial effect.
A controlled clinical trial from Sweden compared the efficacy of metronidazole with sulfasalazine in the treatment of patients with active Crohn’s disease.453,566 Seventy-eight participated in the study during two 4-month periods. Metronidazole was demonstrated to be slightly more effective than sulfasalazine. Although metronidazole may be beneficial for some patients with intestinal disease, some have shown that it does not appear to have therapeutic potential for preventing relapse of Crohn’s disease.18 In other studies, metronidazole appears to be of modest value in preventing postoperative recurrence or maintaining remission, but it is limited by bacterial resistance and drug intolerance, particularly the neuropathic side effects and lack of response at the 3-year mark.56,468 When it has been added to ciprofloxacin, there has been an improvement through the reduction of the CDAI. Forty-five percent of patients went into remission in both the Italians’ and the Canadians’ experiences, with better results noted with colonic or ileocolonic disease as opposed to ileal disease alone.194,424 Patients undergoing prolonged treatment need to be closely monitored for the development of peripheral neuropathy.125
Prolonged antibiotic therapy (i.e., 6 months) appears to be of value in cipro-treated patients by reducing their CDAI compared with placebo.25 It is not surprising because many clinicians maintain patients on antibiotics for years with ileocolonic Crohn’s.
Conclusions regarding clinical trials of antibiotics support a role for metronidazole-imidazoles, cipro, and possibly clarithromycin in Crohn’s colitis and ileocolitis, and less so for ileitis. Better data are still needed with respect to efficacy, recognition of the toxicity of metronidazole, and the expense of other medications.
Metronidazole has been suggested as being particularly useful in the management of anal and perianal Crohn’s disease (see Chapters 13 and 14).42,56,130 Bernstein and associates demonstrated that drainage, erythema, and induration diminished dramatically in all 21 patients so treated, with complete healing obtained in more than one-half of those who were maintained on therapy.42 Eisenberg compared surgery alone in the management of complicated anal Crohn’s disease with surgery plus metronidazole.130 The patients were randomly allocated, but it was not a double-blind study. An intravenous loading dose of 15 mg/kg (usually 1 g) was given, with maintenance therapy of 500 mg intravenously every 6 hours for 5 days. Outpatient treatment was continued at the dosage of 250 mg, three times daily, for 4 weeks or until complete healing occurred. Twelve of 14 patients (86%) receiving metronidazole treatment had a satisfactory response—complete healing of anorectal-perineal disease without relapse during a follow-up period of 1 to 5 years. In the control group, 11 of 17 patients (65%) treated by surgery alone had satisfactory healing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree