Carlos R. Estrada, Jr., MD, Lynne R. Ferrari, MD A baby born before 37 weeks’ gestation is considered premature. The severity of prematurity may be indicated by the birth weight, although these two factors are not necessarily related. Infants weighing 2500 g or less at birth are considered low birth weight (LBW) in prematurity, but in an infant born full term, this weight would indicate intrauterine growth restriction (IUGR). This is an important distinction, because full-term neonates with IUGR usually have different problems than premature infants. In the United States, approximately 30% of LBW infants are born after 37 weeks’ gestation. Premature infants weighing 1500 to 2500 g are referred to as moderately low birth weight (MLBW); those less than 1500 g are referred to as very low–birth-weight (VLBW) infants; and infants who weigh less than 1000 g are considered extremely low–birth-weight (ELBW) infants. MLBW infants account for 82% of premature infants, VLBW 12%, and ELBW 6%. In 2006, 12.8% of live births in the United States were premature, and 8.3% were LBW. Between 1996 and 2006, the rate of premature births in the United States increased more than 16%. With respect to race, the rate of prematurity in the United States is highest for black infants (18.3%), followed by Native Americans (14.1%), Hispanics (12.1%), whites (11.6%), and Asians (10.7%). The clinical implications of prematurity are profound, and premature infants accounted for 16.6% of all infant deaths in the United States in 2005. These implications are especially relevant to the VLBW and ELBW infants, who are surviving in increasing numbers due to remarkable advances in neonatal critical care. These infants account for a large proportion of neonatal deaths and long-term disability, and because of their extreme prematurity are predisposed to hyaline membrane disease, leading to chronic lung disease, retinopathy of prematurity, intraventricular hemorrhage, and necrotizing enterocolitis (Teitelbaum and Coran, 2003b; Pierro, 2006; Eichenwald and Stark, 2008; Goldenberg et al, 2008). IUGR is defined on prenatal ultrasonography as a fetus whose estimated weight is below the 10th percentile for its gestational age. At term, the birth weight below 2500 g is considered IUGR. Approximately 70% of infants with a birth weight that qualifies for IUGR are constitutionally small, and in the remaining 30%, the cause of IUGR is pathologic. IUGR can be limited to intrauterine development, resulting in a normal-sized baby at birth. General causes of IUGR include placental insufficiency, chronic maternal disease, abnormal placentation, genetic disorders, malformations, immunologic diseases, maternal infections, metabolic diseases, maternal substance abuse, and multiple gestations. IUGR is typically classified as symmetrical or asymmetrical. Symmetrical IUGR describes a fetus whose entire body is proportionally small, and is considered the more severe form (Styne, 2004). Asymmetrical IUGR is related to processes that require the fetus to direct its energy to the maintenance of vital organs, usually the heart and brain. Therefore the fetus with asymmetrical IUGR typically has a normal head circumference but small abdominal circumference, small limbs, reduced skeletal muscle mass, and decreased subcutaneous and abdominal fat. Infants with asymmetrical IUGR more frequently exhibit catch-up growth than their symmetrical IUGR counterparts. However, of all infants with IUGR, 10% to 30% will have short stature as adults. Given the wide variety of causes, the management of IUGR is individualized, and often complex decisions are required, weighing elective preterm delivery and the risks of prematurity against the risks associated with IUGR (Teitelbaum, 2003b; Pierro, 2006; Alberry and Soothill, 2007; Goldenberg et al, 2008). The embryonic stage begins with a ventral bud from the foregut at 3 weeks’ gestation, which divides into the primordial segments that give rise to all lung tissue (Teitelbaum, 2003b; Wilson, 2006). The fetal stage of lung development begins at 7 weeks’ gestation and proceeds to term. This stage is further subdivided into three phases: pseudoglandular (7 to 17 weeks), canalicular (16 to 25 weeks), and saccular (25 weeks to term). The pseudoglandular phase is of particular interest, because its timing coincides with the replacement of placenta-derived amniotic fluid with fetal urine-derived amniotic fluid. By the end of the 16th week of gestation, all lung branching occurs, resulting in the terminal bronchial airways. After this time, the only further growth that occurs is elongation and widening of existing airways. A large body of experimental data indicates that these early and critical events in lung development are dependent on lung fluid dynamics, and any restrictive process, including tracheal occlusion (e.g., atresia) or oligohydramnios, results in pulmonary hypoplasia, which can be fatal at birth. The severity of the restrictive process is proportional to the degree of hypoplasia (Teitelbaum, 2003b; Wilson, 2006). The postnatal stage continues for one year after birth. This stage is characterized by maturation of the terminal saccules into alveoli. At birth, the lung contains approximately 20 million saccules, and at approximately 5 weeks postnatal, these begin to develop into the 300 million alveoli expected to be present by 8 years of age. The most robust development of the alveoli occurs before 4 years of age. After 8 years of age, lung volume increases because of increase in alveolar size, not from an increase in alveolar number (Teitelbaum, 2003b; Wilson, 2006). In the postnatal period, growth and development in children occurs at a rapid pace, especially in early childhood. A full-term newborn grows at a rate of 25 to 30 g/day over the first 6 months of life, leading to a doubling of the birth weight during this period. In the first 12 months of life, an infant’s birth weight is typically tripled. By 3 years of age, birth weight is expected to quadruple, and by 10 years of age it will increase 20-fold from the birth weight. Body length increases by approximately 50% in the first year of life, and by threefold by 10 years of age (Teitelbaum and Coran, 2003c). The neonatal and pediatric myocardium is stiffer and less compliant compared with the adult heart. This results in diminished preload capacity, so that increases in end-diastolic ventricular volume and increases in right ventricular pressure result in decreased cardiac output at lower levels than in adult patients. In addition, infants and children have relatively higher resting heart rates. As a result, cardiac output in children is heart rate dependent, because the stroke volume is relatively fixed. Decreases in heart rate in infants and children will result in decreases in cardiac output to a greater extent than a similar decrease in heart rate in an adult patient. A reduction of a child’s heart rate to that of a typical adult would result in marked decrease in cardiac output. Finally, the pediatric heart is significantly less responsive to inotropic agents, because it has reduced intramyocardial calcium release (Hirschl, 2003a; Rocchini, 2006). Congenital heart defects are very common, occurring in approximately 1 of every 120 live births. In general, they are classified as hypoplastic, septal, cyanotic, or obstruction defects. The septal defects are the most common, of which ventricular septal defect (VSD) is most prevalent. Most infants with septal defects have no symptoms during the first month. However, after 4 to 6 weeks of life, pulmonary resistance reaches normal levels, and thus during the second month of life, congestive heart failure can occur. Hypoplasia of the heart is rare, but is the most serious form of congenital heart disease. These defects typically result in the failure of either the right ventricle or the left ventricle to develop adequately, leaving only one side of the heart capable of pumping blood to the body and lungs. In hypoplastic left heart syndrome, the presence of a patent ductus arteriosus is critical for the infant’s ability to survive until emergency surgery can be performed. Without this pathway, blood cannot circulate to the body. In hypoplastic right heart syndrome, a patent foramen ovale serves the same function. Obstruction defects occur when heart valves, arteries, or veins are abnormally narrow or blocked. Common obstruction defects include pulmonary valve stenosis, aortic valve stenosis, and coarctation of the aorta, with other types, such as bicuspid aortic valve stenosis and subaortic stenosis, being comparatively rare. Any narrowing or blockage can cause enlargement of the heart or hypertension. Cyanotic heart defects are named thus because they result in cyanosis. These defects include persistent truncus arteriosus, total anomalous pulmonary venous connection, tetralogy of Fallot, transposition of the great vessels, and tricuspid atresia. From a noncardiac surgery perspective, it is important to remember that many children with complex cardiac anomalies are on medications, such as aspirin and sildenafil, that predispose them to bleeding. In addition, certain types of surgically induced circulation—for instance, induced by the Fontan procedure—purposely increase systemic venous pressure, which can be problematic for postoperative bleeding. Careful consideration of these variables and planning with a pediatric cardiac anesthesiologist is obligatory (Hirschl, 2003a; Rocchini, 2006). Although the number of neutrophils is near the adult level at term (approximately 60% of circulating leukocytes), neonates have a relative inability to increase their circulating levels in response to stress or infection. This is thought to be due to a decreased neutrophil storage pool and to increased margination of neutrophils. Premature infants have the added problem of having a significantly lower neutrophil count at birth. Neonatal neutrophils are also less adhesive to activated endothelium, a process that is critical to chemotaxis and migration to sites of inflammation and infection. This may be due to decreased neonatal neutrophil expression of L-selectin and β2 integrin. In addition, neonatal serum is deficient in opsonins, which are necessary for neutrophil phagocytosis. Therefore even though neonatal neutrophils are fully competent to kill bacteria, they may do so less efficiently. Unlike a decreased neutrophil storage pool, the number of monocytes in neonates is equal to or greater than in adults. For unclear reasons, however, the migration of monocytes to sites of inflammation and infection is significantly delayed (Hirschl, 2003b; Upperman, 2006). T-lymphocyte function is also impaired in neonates, despite having a significantly greater number of circulating T cells compared with adults. In addition, unlike adults, there is a greater proportion of CD4+ T cells than CD8+ T cells. The impaired function is believed to be related to their naive phenotype that is due to their lack of exposure to foreign antigens and because they produce relatively limited amounts of key inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin (IL)-3, IL-4, IL-5, and IL-10. B-lymphocyte function is also impaired in neonates, which is due to their inability to differentiate into immunoglobulin (Ig)G- or IgA-secreting plasma cells. They are able to differentiate into IgM-secreting plasma cells but rely on maternal placental transfer for essentially all IgG. This reliance continues until the third or fourth month of life, after which time the proportion of neonatal IgG overtakes maternal IgG. Although maternal IgG is adequate for protection against most infections, strains of bacteria, such as Escherichia coli and Salmonella, can elicit a different immunoglobulin subtype, leaving the fetus and neonate with suboptimal immune protection. Premature infants are at even greater risk, because they are born without sufficient levels of maternal IgG (Hirschl, 2003b; Upperman, 2006). Another lymphocytic deficiency in neonates involves the natural killer (NK) cells, which play an important role in protection against intracellular pathogens by targeted cell lysis. At term, the proportion of NK cells is similar to that in adult circulation, but they are functionally and phenotypically immature. For unclear reasons, their lytic potential is only 50% of adult NK cells, and it is not until late infancy that they reach their full functional potential (Hirschl, 2003b; Upperman, 2006). The relative deficiency of immunoglobulins in neonates results in the increased reliance on the alternative, that is, antibody-independent pathway of complement activation. However, in neonates there are a reduced number of classic and alternative complement activation pathway factors. Specifically, the level of C9 is diminished, which is critical for protection against gram-negative bacteria. Breast-feeding is believed to partially compensate for these intrinsic neonatal immunologic deficiencies. Human milk contains immunoglobulins, including IgG, IgM, and secretory IgA; lymphocytes; macrophages; polymorphonuclear leukocytes; and components of the complement cascade. Because of these and other benefits, the American Academy of Pediatrics recommends continuing breast-feeding for the first 12 months of life. Although the neonatal period presents the highest risk of infection for children, the immune system is not fully competent until approximately 8 years of age (Hirschl, 2003b; Upperman, 2006). A detailed discussion of renal development can be found in Chapter 112 of this text, and therefore this section represents a very brief synopsis. Renal function begins in utero, with the first functional nephrons appearing at 8 weeks’ gestation. Nephrogenesis is complete by 34 weeks. Urine production begins between 10 and 12 weeks’ gestation, coinciding with the start of glomerular filtration. In utero, renovascular resistance is high, limiting renal blood flow. Immediately following birth, the distribution of renal cortical blood flow changes, with increased perfusion of the outer cortex and increased reactivity of the renal vascular bed. Consequently, the glomerular filtration rate (GFR) rises quickly despite renal blood flow remaining unchanged. In addition, water and electrolyte homeostasis is difficult to predict. GFR and tubular function double by 1 month of age (Kaskel et al, 1987), and over the first 3 months of life, renovascular resistance continues to decrease, which results in further rises in GFR. Following this relatively rapid rise, GFR continues to increase more slowly toward adult levels, which are reached by 12 to 24 months of life. The maturation of renal tubular function lags behind the maturation of glomerular function, and therefore the neonate can concentrate urine to only approximately 50% of adult capability (Greco, 2002; Teitelbaum and Coran, 2003a; Pierro, 2006). Maintenance fluid replaces two losses: insensible, or evaporative, losses and urinary losses. In the perioperative period, insensible losses can vary widely with the presence or severity of several variables, including fever, tachypnea, and other conditions. Insensible losses represent loss of free water and generally account for one third of maintenance fluids. Urine losses are calculated as 280 to 300 mOsm/kg of water with a specific gravity of 1.008 to 1.015, but this concentration can vary depending on the patient’s ability to concentrate urine. Urinary losses account for two thirds of total maintenance fluids. The total requirements for maintenance fluids can be calculated using the Holliday-Segar formula as shown in Table 119–1 (Holliday and Segar, 1957). After calculating the fluid requirement, children usually receive either 5% dextrose (D5) Table 119–1 Daily Maintenance Fluid Requirements From Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957;19:823–32. In the setting of postoperative dehydration, the severity is determined as described in Table 119–2 (Siker, 2002). Generally, deficit replacement should begin with a balanced salt solution, such as lactated Ringer (LR) solution or normal saline, to increase intravascular volume. Typically, a bolus of 10 to 20 mL/kg is used, but a rate of up to 40 mL/kg during the first 1 to 2 hours is well tolerated (Carvajal, 1994). The type of fluid deficit can be estimated from the patient’s history, physical examination findings, electrolyte values, and serum tonicity. Types of dehydration include isotonic (serum osmolarity 270 to 300 mOsm/L, serum sodium [Na+] concentration 130 to 150 mEq/L), hypotonic (serum osmolarity < 270 mOsm/L, serum Na+ concentration < 130 mEq/L), or hypertonic (serum osmolarity > 310 mOsm/L, serum Na+ concentration > 150 mEq/L). Patients with hypertonic dehydration require careful consideration of fluid type and rate, because complications, such as cerebral edema, may occur during rehydration (Friedman, 2005; Greenbaum, 2007). Table 119–2 Estimation of Fluid Deficit Modified from Siker D. Pediatric fluids, electrolytes, and nutrition. In: Gregory GA, editor. Pediatric anesthesia. New York: WB Saunders; 2002. Key Points Perioperative Fluids The psychologic state of a child and family, as well as the clinical state of each child, must be thoroughly understood before anesthesia and surgical intervention. If the child is not treated in an age-appropriate manner, the entire perioperative experience will likely be compromised. Conversely, if the psychologic and emotional aspects of a child’s condition distract caregivers from the primary medical and surgical concerns, a successful outcome may be compromised. Therefore it is imperative that the entire health care team find the ideal balance between these two considerations (Ferrari, 2008). It is well known that significant preoperative anxiety is associated with a difficult and often prolonged anesthetic induction (Kain et al, 1996a, 1996b). Factors including the temperament and age of the child, as well as the situational distress of the parent and the outcome of previous medical experiences, will affect the child’s anxiety level. For many children, the immediate postoperative course is a mirror of the induction experience. Children who go to sleep peacefully generally awaken in the same manner and are known to have fewer difficulties in the postanesthetic care unit (PACU). It is therefore necessary to take the time to prepare the child for the anesthetic experience in an age-appropriate manner. There is consensus among anesthesiologists regarding the need for the treatment of a child’s anxiety before surgery (McCann and Kain, 2001). The development of coping skills is considered the most effective preoperative intervention, followed by modeling, play therapy, an operating room (OR) tour, and printed material (Kain et al, 1996a; O’Byrne et al, 1997; Moynihan and Kurkar, 1999; Ferrari, 2008). The level of maturity affects a child’s understanding of and response to illness (Moynihan and Kurkar, 1999). Infants fear separation from their primary caregivers and exhibit stranger anxiety; therefore it is important that parental involvement in the perioperative experience be maintained. Toddlers fear loss of control, so enabling a child to make choices, such as asking if the child has a color preference for his or her hospital gown, will diminish anxiety. Preschool-age children fear injury; they may fear, for example, that a blood draw may result in not enough blood being left in their bodies. They tend to think in concrete terms and therefore may take statements literally, so one must be cautious when choosing the language used with this age group. The school-age child typically fears that he or she may not meet the expectations of adults. They may nod with understanding and listen intently, despite not grasping what the adult is saying. They are reluctant to ask questions for fear that they should already know the answer. It is therefore imperative that expectations are clearly explained. Adolescents fear death and usually do not understand bodily functions. They are often panic-stricken preoperatively but try to not show any sign of this. As a result, they might remain very quiet. It is the responsibility of the care team to anticipate this anxiety and reassure the adolescent without prompting (Ferrari, 2008). Most parents will express that they experience more anxiety about the anesthetic than the risks of the surgery. Fear of anesthesia among parents originates largely from a lack of information regarding modern anesthesia practice rather than from a high probability of risk. For many families, it may be helpful to discuss specific risks of anesthesia for their child (Olsson and Hallen, 1984; Ferrari, 2008). For a healthy child undergoing uncomplicated surgery, the risk of an adverse event is approximately 1 in 200,000 (Eichhorn, 1993). The risk of death under anesthesia is the most feared complication. This risk is 1 in 10,000 for all patients of any age undergoing any surgical procedure (Keenan and Boyan, 1985; Tiret et al, 1986; Holzman, 1994). However, the risk of death directly attributable to the anesthetic approaches zero, although the risk of cardiac arrests due to anesthesia remains approximately 4.5 in 10,000 (Gobbo Braz et al, 2006). The incidence of anesthetic-related complications and death is highest during the first year of life at 43 : 10,000, but this decreases dramatically during the second year of life to 5 : 10,000 (Tiret et al, 1988). Anesthetic risks increase by a factor of 6 during emergency procedures in all age groups (Ferrari, 2008; Holzman, 2008). A complete medical history is always the first step in a thorough preoperative assessment. The history should include the prenatal course and neonatal period, because events during pregnancy and delivery may influence the child’s current state of health (Means, 1997). Any prior hospital admissions should be noted. A complete review of systems is performed to evaluate for medical comorbidity that could influence the choice or outcome of anesthesia (Cote, 2001). The presence of cough, asthma, or a recent upper respiratory infection (URI) might predispose the child to bronchospasm, atelectasis, or pneumonia. New-onset heart murmur, cyanosis, hypertension, exercise intolerance, or a history of rheumatic fever can suggest an evolving issue that could be exacerbated by an anesthetic or with a surgical procedure. Parents should be queried for the presence of vomiting, diarrhea, malabsorption, black stools, gastroesophageal reflux, or jaundice to interrogate for electrolyte imbalance, dehydration, hypoglycemia, anemia, or the need for a rapid-sequence induction. A history of seizures, head trauma, or difficulty swallowing may indicate a metabolic derangement, increased intracranial pressure, or sensitivity to muscle relaxants. Urinary tract abnormalities may impact the state of hydration and renal function, and the urologist should clearly communicate the significance of these. Abnormal development, alterations in serum glucose levels, or a history of chronic steroid use may indicate an endocrinopathy, diabetes mellitus, hypothyroidism, or adrenal insufficiency. Lastly, a history of anemia, bruising, or excess bleeding may suggest a transfusion requirement or coagulopathy (Ferrari, 2008). The family history should be obtained with particular attention to anesthesia-related events. Specifically, a history of liver problems in family members after anesthesia is important to elicit, because certain anesthetic agents are known to rarely cause liver damage. Malignant hyperthermia (MH) is always a concern in the pediatric population. Although most pediatric anesthesiologists refrain from routinely using succinylcholine, a family history of prolonged paralysis or mechanical ventilation after general anesthesia should be obtained. Finally, families should be asked if there is a history of unexpected death, sudden infant death syndrome, genetic defects, or familial conditions, such as muscular dystrophy, cystic fibrosis, sickle cell disease, bleeding tendencies, or human immunodeficiency virus infection (Ferrari, 2008). Obtaining a complete medication history is essential. This includes prescription medications, nonprescription medications, and herbal or alternative therapies. Many over-the-counter cold remedies contain aspirin, nonsteroidal anti-inflammatory drugs, or other compounds that may interfere with coagulation. Herbal and alternative therapies are known to have significant deleterious interactions with certain prescription medications and must be considered prior to administration of anesthesia and proceeding with surgery (Cupp, 1999). The practice of body piercing is becoming increasingly common in adolescents and young adults. Metal objects in the skin during surgery and anesthesia increase the risk of burn injury if there is an intraoperative electrocautery malfunction. In addition, metal objects can become caught on equipment in the OR, resulting in tears of the skin and subcutaneous tissue. Tongue piercing may interfere with laryngoscopy and make securing the airway unnecessarily challenging. Therefore patients should be counseled to remove all metal objects and disclose any body piercing that cannot be seen during the preoperative interview (Ferrari, 2008). The physical examination of children must begin with simple observation from a distance, because the infant or child may become frightened when approached directly. A great deal can be learned about relevant physical findings without touching the child. The color of the skin, including the presence of pallor, cyanosis, rash, jaundice, unusual markings, or prior surgical scars, may reveal the presence of organ system dysfunction. Because congenital anomalies often occur in association with others, abnormal facies may indicate additional anomalies. The respiratory system examination should specifically address any signs of an URI. The cardiovascular examination specifically addresses the presence of heart murmurs, which must be accurately diagnosed as innocent versus pathologic. Lesions in which bacterial endocarditis prophylaxis or protection from paradoxic air embolism are required must be documented (Wilson et al, 2007; Ferrari, 2008). Routine diagnostic testing in preparation for surgery is rarely indicated in healthy children, and studies that are ordered should be selected based on the general medical health of the patient and the procedure being performed. In general, measurement of hemoglobin/hematocrit in a healthy child undergoing elective surgery is unnecessary (Steward, 1991). A hemoglobin/hematocrit should be measured if significant blood loss is anticipated or if the child is younger than 6 months or was born prematurely. Neither the routine measurement of a coagulation profile nor a history of “easy bruising” is reliable in predicting surgical bleeding (Burk et al, 1992
Growth and Maturation
Prematurity and Intrauterine Growth Restriction
Intrauterine Growth and Lung Development
Postnatal Considerations
Cardiovascular
Immunologic
Renal
Perioperative Fluids
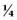 normal saline (NS) + 20 mEq/L potassium chloride (KCl) or D5
normal saline (NS) + 20 mEq/L potassium chloride (KCl) or D5 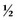 NS + 20 mEq/L KCl. Children who are younger than 6 months are generally given the solution with
NS + 20 mEq/L KCl. Children who are younger than 6 months are generally given the solution with 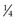 NS because of their high water needs per kilogram. Children 6 months and older, however, should receive the solution with
NS because of their high water needs per kilogram. Children 6 months and older, however, should receive the solution with 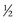 NS (Greenbaum, 2007).
NS (Greenbaum, 2007).
WEIGHT (kg)
DAILY REPLACEMENT
HOURLY REPLACEMENT
0-10
100 mL/kg/day
4 mL/kg/hr
11-20
1000 mL/day + 50 mL/kg/day
40 mL/hr + 2 mL/kg/hr
>20
1500 mL/day + 25 mL/kg/day
60 mL/hr + 1 mL/kg/hr
DEFICIT
CLINICAL FINDINGS
Mild (1%-5%)
Dry mucous membranes, poor skin turgor, irritability, decreased urination
Moderate (6%-10%)
No tears, tenting of skin, lethargy, oliguria
Severe (≥11%)
Sunken eyes and fontanelles, cold skin, anuria, tachycardia and tachypnea, hypotension, coma
Pediatric Anesthesia and Analgesia
Psychologic and Emotional Preparation
Risk of Anesthesia
Basic Preoperative Preparation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Core Principles of Perioperative Management in Children
• Maintenance of appropriate hydration is a fundamental and critically important concept in pediatric care.

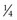 NS + 20 mEq/L KCl or D5
NS + 20 mEq/L KCl or D5 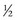 NS + 20 mEq/L KCl, depending on their age and weight.
NS + 20 mEq/L KCl, depending on their age and weight.




