Christopher K. Payne, MD
General Considerations
Impact of Urinary Incontinence
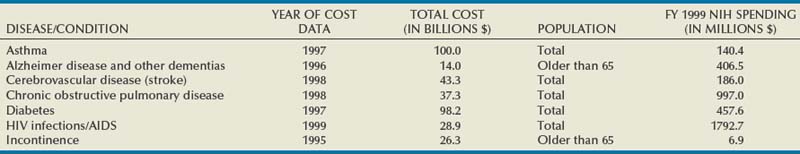
Urinary incontinence (UI) affects people in all strata of society—the young and the old, male and female, rich and poor, all ethnic and racial backgrounds—although women and older individuals bear a disproportionate share of the burden. The impact is enormous. The most recent comprehensive accounting estimated the total annual direct and indirect costs for UI in the United States alone to be $19.5 billion (in year 2000 dollars; Hu et al, 2004). In a 2000 report to Congress, the National Institutes of Health (NIH) estimated the direct cost of UI at $12.5 billion, a figure comparable to other important diseases such as Alzheimer disease, chronic obstructive pulmonary disease, and human immunodeficiency virus infection/acquired immunodeficiency syndrome (Table 69–1). Although medical insurance covers some expenses, patients with UI bear a considerable percentage of the overall disease costs for protective pads, laundry, and other expenses not covered by individual insurance plans. The median out-of-pocket costs to women with weekly or more UI were $186/year (2005 dollars), but those with severe UI spent $372/year and those with very severe UI spent $1148/year (Subak et al, 2007). These costs appear to be increasing rapidly; Medicare expenditures on women with UI increased 57%, adjusted for inflation, between 1992 and 1998 (Anger et al, 2006). As summarized in the Third International Consultation on Incontinence (ICI) Economics Committee (Hu et al, 2005) the consequences of UI lead to serious morbidity, including falls and fractures; urinary tract infections; skin breakdown, including pressure ulcers; and admission to nursing homes. Logically, the presence of lower urinary tract symptoms, including overactive bladder (OAB)/UI symptoms, was found to be associated with approximately 50% increased emergency department visits, hospitalizations, and medical provider visits (Kannan et al, 2009). Despite these figures, and despite markedly increased efforts to inform the public about UI in the past decades, the majority of patients suffer quietly; in one study, fewer than one third of women with bothersome UI in a prepaid health care plan had been diagnosed in the prior 5 years and few had been treated (Kinchen et al, 2007).
The Urologic Diseases in America project has published detailed analyses of expenditures by the U.S. health care system for UI in both men (Stothers et al, 2005) and women (Thom et al, 2005). Among the more interesting findings was a dramatic increase in Medicare expenditures for women from $128.1 to $234.4 million between 1992 and 1998, despite decreasing hospitalizations and length of stay. In men, the prevalence of incontinence was estimated at 17% for those older than 60 years of age (any incontinence over the past 12 months) and the annual expenditures for privately insured male adults with UI were $7702 compared with $3204 for a man without UI. Demographic trends producing increasing numbers of elderly individuals will make this problem a critical challenge to urologists for the coming decades. Those interested in further information are referred to the report of the Epidemiology Committee of the Fourth ICI (Milsom et al, 2009) which provides excellent summaries of prevalence, incidence, remission, and risk factors.
The impact of UI cannot be measured in dollars alone. A “social cancer,” UI impacts every facet—social, physical, sexual, psychological, and medical—of human life at work and at home. Numerous reports continue to document the serious impact of UI and overactive bladder (OAB) on quality of life using high-quality methodology (Coyne et al, 2003, 2004; Avery et al, 2004; Hajjar, 2004). Incontinent patients are more likely to have poor self-esteem, feeling shame and guilt, which may keep them from working effectively and partaking in social activities. UI is strongly associated with depression and vice versa (Steers and Lee, 2001), although the nature of this relationship is not established. Stress urinary incontinence (SUI) restricts the physical activity of patients, many of whom are otherwise healthy young women. The long-term deleterious effects of exercise reduction on health are unclear but may be important. Urgency urinary incontinence (UUI) affects quality of life even more than SUI because of its unpredictable nature. It may lead to loss of sleep as well as the limitations just mentioned. Sexual activity and interpersonal relationships suffer due to UI. Despite this, many and perhaps most individuals still do not seek help for UI for a variety of reasons, including misguided perceptions that UI is a normal consequence of aging and that there is no effective treatment (Shaw, 2001). Even more importantly, expenditures for UI research are a small fraction of that spent on other conditions; NIH-funded UI research is less than 2% than that for stroke or Alzheimer disease (see Table 69–1).
Rationale for Conservative Therapies
This “silent” epidemic was brought to national attention with the 1988 NIH consensus conference (Consensus Conference, 1989) followed by the publication of the first Agency for Health Care Policy and Research (AHCPR) practice guideline on UI in adults in 1992 (Urinary Incontinence Guideline Panel, 1992). Two of the principal recommendations of the guideline relate directly to improving the recognition of UI and validating it as an important medical problem:
A third recommendation of the 1992 AHCPR guideline applies directly to the focus of this chapter:
Description: What is “Conservative,” Who is it for?
To this end, levels of evidence and grades of recommendation are used whenever possible and conclusions drawn from systematic literature reviews and the ICI are highlighted. The AHCPR has used specified evidence levels to justify recommendations for the investigation and treatment of a variety of conditions, and the Oxford Centre for Evidence Based Medicine produced a widely accepted adaptation of the work of the AHCPR (Oxford Centre, 2001). The highest level of evidence (level 1) is based on systematic reviews and/or randomized controlled trials. The highest grade of recommendation (grade A) stems from consistent level 1 evidence. When possible these terms are used in the chapter, although there is a great need for further high-quality research and there is a notable lack of long-term follow-up of treatment effect for almost all of the therapies discussed.
Overview
The challenge to the urologist is to understand all of the treatments for UI, to be able to assess the patient efficiently yet thoroughly, and then to construct an appropriate treatment plan with the patient, taking into account the problem and the goals of the individual patient. A review is presented of the basic nonsurgical tools used in treating UI—behavioral therapy, pelvic floor muscle training and biofeedback, external devices, and peripheral electrical and magnetic stimulation. Pharmacologic therapy is discussed in Chapter 68, surgical and injection therapy in Chapters 71 to 74, and neuromodulation in Chapter 70. The detailed evaluation of the incontinent patient is discussed in Chapter 64. Although it is important to rule out serious underlying or associated conditions, invasive testing is rarely required before initiating treatment with the measures discussed here. The focus here is on only that part of the evaluation that directly relates to treatment planning. It is generally sufficient to have a working diagnosis classifying the patient as having stress, urgency, or mixed UI. Patients with symptoms of OAB (the generic term for urgency and frequency with or without UI) are treated in the same manner as those with UUI. Practical algorithms are presented that provide a rational means of applying these varied treatments to an individual patient.
The Tools of Conservative Therapy
Behavioral Therapy
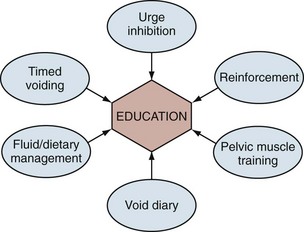
Behavioral therapy describes a group of treatments grounded in the concept that the incontinent patient can be educated about his or her condition and develop strategies to minimize or eliminate UI. It is sometimes erroneously reduced to the combination of fluid restriction and timed voiding but is actually a far richer therapy. In fact, one of the main problems with the term behavioral therapy is that it has been used so differently by various practitioners that its meaning has become diluted and vague. There is no one standard protocol or “best” methodology. The shared aims of behavioral therapy are illustrated in Figure 69–1 (Payne, 2000). Although various practitioners may have different emphasis, the different treatment approaches are unified by education about normal urinary tract function. The individual elements of behavioral therapies discussed here are centered on basic educational techniques such as operant learning, which is intended to model activity so as to reproduce normal behavior, in this case urinary continence (Palmer, 2004). All of the individual techniques discussed fall into this category, although pelvic floor muscle training is both a behavioral therapy (education about anatomy and function of the muscles, learning to use the muscles properly to control lower urinary tract function) and a physical therapy (strengthening the muscles to improve function). In any case, education binds the various techniques together and plays the central role in behavioral therapy.
Bladder Training/Timed Voiding
The term scheduled voiding is the generic term preferred for describing voiding regimens used for home-dwelling cognitively intact patients as opposed to prompted voiding or toileting, terms properly applied to institutionalized or otherwise dependent patients. The most commonly used technique for patients with OAB and UUI is “bladder training” (“bladder drill,” “bladder retraining”). Bladder training starts a patient voiding on a fixed time interval schedule with the intention that, most of the time, the patient will urinate before experiencing urgency and UI. The interval is gradually increased with clinical improvement. Early practitioners of bladder training first established the effectiveness of intensive inpatient bladder training temporarily supplemented by medications (Frewen, 1978). Next, outpatient treatment was proven to be effective (Elder and Stephenson, 1980; Frewen, 1980). Finally, the durability of response, with 85% initial and 48% three-year response rates was reported (Holmes et al, 1983). Bladder training should always be combined with urge inhibition techniques and is often combined with anticholinergic medical therapy, particularly for more severe cases and for patients with neurogenic bladder. In contrast, “timed voiding” involves having a patient void on a fixed schedule, typically every 2 to 3 hours, and is intended to normalize frequency in a patient with infrequent voiding and/or diminished bladder sensation. This technique can be employed for patients with SUI with the idea that leakage will be less if the bladder is less full when physical stress occurs. It can also be used in a variety of patients with UUI who have a good bladder capacity (the classic example is that of patients with diabetic neurogenic bladder; they do not have proper bladder sensation and thus delay voiding inappropriately).
Although there is no evidence as to the optimal program of bladder training, a common approach is to start at a safe interval based on the patient’s bladder diary and increase the interval by 15 to 30 minutes as the patient achieves continence. The ultimate goal is a comfortable interval between voids with continence—a “retraining” of the bladder. Wilson and colleagues (2005) concluded that if no improvement is made after 3 weeks “the patient should be reevaluated and other treatment options considered.” The ICI authors acknowledge that the quantity of evidence is low but still make a grade A recommendation that “bladder training is recommended as a first line treatment of UI in women” (Hay-Smith et al, 2009). Medical therapy is commonly employed initially, and there are rather limited studies comparing bladder training to anticholinergic medications for detrusor overactivity and UUI. One study demonstrated superior results with the combination of behavioral therapy and anorectal biofeedback in comparison to anticholinergic medication alone (oxybutynin chloride in titrated dosing) in a group of patients with urge and mixed UI (Burgio et al, 1998). Subsequent work by Burgio and colleagues (2000) confirms the clear truth that behavioral and drug therapy are complementary, not competitive treatments; the primary value of this research is to underscore the value of the behavioral component in the treatment plan. Logically, all patients should have behavioral therapy with medications used on an individualized basis. The ICI committee (Hay-Smith et al, 2009) found level 2 evidence that the effect of bladder training may be enhanced by drug therapy and that, for women already taking an antimuscarinic drug, there was no additional benefit from adding brief written instructions on bladder training.
Smoking
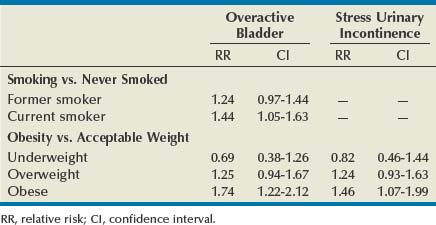
Aside from being a major risk factor for bladder cancer, smoking has been proposed as a risk factor for SUI by increasing coughing episodes and for OAB through bladder irritation from nicotine and toxins excreted in the urine. However, to date, epidemiologic studies of tobacco use have produced inconsistent findings. In women, some studies suggest that smoking increases the risk of UI, or at least severe UI, but others demonstrate no increased risk. A 1-year longitudinal study of 6424 women older than 40 years of age found that current smokers were at higher risk for both SUI and OAB than those who had never smoked, although statistical significance was seen only for OAB (Table 69–2). Former smokers had intermediate risk (Dallosso et al, 2004a). The same group also published a longitudinal study of 4887 men showing no association between smoking and OAB symptoms (Dallosso et al, 2004b). Another population-based study suggested that the risk of UI is limited to subjects with a history of more than 15 pack-years or current consumption of more than 20 cigarettes a day (Hannestad et al, 2003). One group of researchers found that smoking was associated with the severity of UI in women seeking surgical treatment (Richter et al, 2005). As of yet there have been no adequate studies of smoking cessation on prevention or treatment of UI symptoms, and the ICI committee could make no evidence-based recommendation (Hay-Smith et al, 2009). Despite these conflicting results, smoking cessation can logically be recommended as a general health measure, to reduce the risk of bladder cancer, and for those smokers with SUI particularly related to coughing. More research, particularly on the effectiveness of smoking cessation in treating UI and on the relationship between smoking and OAB, would be welcomed.
Caffeine
Caffeine is well known as a nervous system stimulant and has demonstrable effects on detrusor muscle in vivo and in vivo, promoting detrusor overactivity. In one study of community-dwelling elderly women subjects who decreased caffeine and increased fluid intake, increased voiding volumes and fewer accidents were experienced (Tomlinson et al, 1999). High caffeine intake (>400 mg/day average) also correlated with urodynamic detrusor overactivity compared with stress-incontinent women (<200 mg/day average) (Arya et al, 2000). Caffeine has thus been postulated to be a cause of OAB symptoms, and caffeine reduction has been advised for OAB patients. Epidemiologic data are less clear. Reports usually break down consumption by type of beverage rather than actual caffeine consumption as is typically found in smaller studies of dietary intervention. Such small studies have typically shown a correlation between caffeine reduction and reduction of UI (Tomlinson et al, 1999; Bryant et al, 2002). Although larger studies would be helpful, it seems appropriate to recommend restriction, particularly in those patients with very high intake of caffeine. The ICI committee concluded that, “while large cross-sectional surveys indicate no association (level 3 evidence), small clinical trials do suggest that decreasing caffeine intake improves continence (level of evidence 2)” (Hay-Smith et al, 2009).
Fluid Management
Fluid restriction has been advocated in the treatment of both SUI and OAB. The rationale is that abdominal leak pressures appear to be volume dependent; therefore, physical stress occurring at lower bladder volumes both will be less likely to cause UI and will be associated with lower volume loss when leakage does occur. Similarly, OAB is believed to be a volume-driven phenomenon and slower filling promotes bladder compliance and lower pressures. This concept appears to be well accepted, as manifest by a recent U.S. study in which 38% of incontinent women had tried limiting fluids compared with 21% who tried Kegel exercises and 6% using prescription medications (Diokno et al, 2004a). On the other hand, extreme fluid restriction produces concentrated urine, which has been postulated to be a bladder irritant, leading to detrusor overactivity as well as constipation, which can negatively affect bladder function. Indeed, there is conflict regarding fluid management, with some investigators showing improvement with fluid reduction (Swithinbank et al, 2005) and others finding that increasing fluid intake improved UI (Dowd et al, 1996). Hashim and Abrams (2008) studied this controversy using a crossover trial in which patients with OAB were instructed to first decrease fluid intake 25% to 50% below baseline and then to increase intake 25% to 50% above the baseline (or the reverse). A significant improvement in continence was noted with decreasing fluids (and increased UI was noted with increasing fluids). It certainly seems reasonable to obtain a baseline frequency-volume chart and advise those patients with normal to increased fluid intake to try moderately restricting fluid intake. Any potential benefit must be balanced against possible problems with bladder infections and constipation.
The types of fluid intake may also be significant; caffeinated beverages, acidic juices, and alcohol have been suggested to be bladder irritants. Caffeine was discussed earlier. Tea consumption correlated with UI in the EPINCONT study, but alcohol and coffee consumption did not (Hannestad et al, 2003). Although it is possible that caffeine is not the critical component, there is no clear hypothesis as to why tea (which has less caffeine than coffee) would be a relevant dietary factor but not coffee. Unless confirmed in other studies this may turn out to be a statistical aberration. In a population of Chinese women, alcohol consumption correlated with SUI but not UUI (Song et al, 2005), but other studies have shown no link between alcohol use and UI symptoms. Epidemiologic data support a link between consumption of carbonated beverages with both SUI and OAB (Dallosso et al, 2003) with level 2 to 3 evidence. Data on the effects of other beverages are scarce.
Other Dietary Management
A variety of different dietary maneuvers have been recommended for incontinent patients, including avoidance of alcohol, carbonated beverages, acid foods, salt, and others. A detailed study examining dietary factors noted an association between intake of vegetables with lower risk of OAB and intake of fruit with lower risk of SUI (Dallosso et al, 2003). These observations have yet to be reproduced in other populations. A follow-up report (Dallosso et al, 2004a) examined details of the diet in relationship to SUI and found that “intakes of total fat, saturated fatty acids and monounsaturated fatty acids were associated with an increased risk of SUI onset one year later. Of the micronutrients studied, zinc and vitamin B12 were positively associated with SUI onset.” Because these observations are not hypothesis driven, confirmation is mandated before any recommendation can be given. No similar connection was identified with alcohol consumption in the same or other studies, and one study actually suggested a lower risk of OAB in men who were beer drinkers (Dallosso et al, 2004b). Once again, the best advice for incontinent patients would seem to be to follow established guidelines for overall health with moderation in alcohol use and adequate intake of fruits and vegetables.
Obesity/Weight Reduction
Epidemiologic data provide persuasive support for a causal association between obesity and UI. The Nurses’ Health Study identified 6,790 women with incident UI over a 2-year period among 35,754 women initially reporting no UI (Townsend et al, 2008a). There were highly significant trends of increasing risk of UI with increasing BMI and waist circumference (P for trend < .001 for both). The same research group showed that not only was obesity a risk for UI but also that weight gain was an independent risk for incident UI (Townsend et al, 2007). Gaining 5 to 10 kg after age 18 increased the risk of developing weekly UI by 44% (OR 1.44, CI 1.05 to 1.97) compared with women who maintained their weight within 2 kg—regardless of the initial weight! Gaining 30 kg increased the risk fourfold. The relationship between obesity, weight gain, and incident UI held for both SUI and UUI. Another powerful study from the United Kingdom followed 1201 women from their birth in 1946 annually from 48 to 54 years (Mishra et al, 2008). At the age of 20, 26, 36, and 43, body mass index (BMI) was positively associated with stress symptoms and severe UI in midlife and there appeared to be a cumulative effect. These relationships existed even after accounting for the effects of aging, childhood enuresis, childbirth characteristics, menopause, educational attainment, and smoking status. Interestingly, these researchers found that BMI was not significantly associated with symptoms of UUI. Finally, in a 1-year longitudinal study of 6424 women older than 40 years of age there was a strong correlation between BMI and the risk of both OAB and SUI (Dallosso et al, 2003) (see Table 69–2). Work in other populations consistently confirms the relationship between obesity and UI (Thom et al, 1997; Brown et al, 1999; Fornell et al, 2004; Larrieu et al, 2004; Melville et al, 2005b). The work in this field provides consistent findings describing a linear relationship between obesity and UI that cumulatively produce conclusive evidence for obesity as an important modifiable risk factor for UI.
More importantly, it is now clear that weight loss is an effective treatment for UI. A prospective randomized controlled trial (RCT) studied 338 overweight and obese women at two centers in the United States (Subak et al, 2009). All had BMI greater than 25 (mean 36) and at least 10 UI episodes per week (>75% with mixed UI). Subjects were randomized to an intensive 6-month weight loss program that included diet, exercise, and behavior modification (226 patients) or to a structured education program (112 patients). Weight loss was moderate (−8 kg vs. −1.6 kg), yet statistically significant decreases were found for all UI (−47% vs. −28%) and SUI (−58% vs. −33%). There was a trend to reduction in UUI (−42% vs. −14%, P = .14). More patients rated themselves to be moderately or very satisfied with the change in UI (75.8% vs. 46.8%, P < .001). These strong results were mirrored in a 2-year open-label trial in the United Kingdom of 64 obese women who were offered a commercially run program of diet and exercise (Rosemary Conley Diet and Fitness Clubs) aiming for weight loss of 5% to 10% over 6 months (Auwad et al, 2008) and by the initial pilot RCT by Subak and colleagues (2005). The high value of bariatric surgery for morbidly obese patients with UI has been clearly documented in four different case series, all showing dramatic reduction in UI episodes (Burgio et al, 2007; Kuruba et al, 2007; Maher et al, 2008, Laungani et al, 2009) (Table 69–3).
Other Lifestyle Issues
As summarized by Wilson and associates (2005), “there are many other lifestyle interventions suggested either by health care professionals or the lay press for the treatment of UI, including reducing emotional stress, wearing non-restrictive clothing, utilizing a bedside commode, decreasing lower extremity edema, treating allergies and coughs, wearing cotton underwear, and increasing sexual activity. These interventions are, however, all anecdotal in nature.”
It has been shown (level 2 evidence) that stress-induced urine loss can be reduced by positional changes, that is, having a woman cross her legs with coughing, although this was only examined in the acute urodynamic testing situation (Norton and Baker, 1994). The ICI panel (Hay-Smith et al, 2009) concluded that constipation and chronic straining may be a risk factor for the development of UI (level 3 evidence) but that there were no data on the effect of intervention.
There is inadequate knowledge about the relationship of strenuous activity, sports, and work. It has long been known that low-grade SUI is common in female college athletes (Nygaard et al, 1994). However, the long-term clinical significance of this is unknown. Because women are universally advised to restrict activity after surgical treatment for SUI one might assume that the effect of postoperative activity on outcome had been investigated, but there are actually no useful data. One study has examined the association between physical activity and risk of developing UI by analysis of data from incident cases of UI in the Nurses’ Health Study of women aged 54-79 years (Danforth et al, 2007). The authors found that increasing levels of total physical activity (which was mainly walking) were significantly associated with a reduced risk of UI and SUI although not with UUI (Table 69–4). The same researchers examined the dataset to explore the relationship for younger women, age 37 to 54 (Townsend et al, 2008b). They again found that activity was inversely correlated with incident UI and that the protective effect of exercise appeared to be present for both SUI and UUI. Accounting for the independent effect of BMI attenuated the findings, but exercise remained a significant independent factor. Although it is possible that these data may not apply to women engaged in very high level, chronic, strenuous activity, the important lesson is that good health habits clearly correlate with a lower risk of UI.
Table 69–4 Physical Activity and Urinary Incontinence
| Any Incontinence | ||
|---|---|---|
| Cases* | OR† (95% CI) | |
| Physical Activity (MET-hr/wk) | ||
| Quintile 1 | 524 | Referent |
| Quintile 2 | 534 | 1.04 (0.92-1.18) |
| Quintile 3 | 465 | 0.90 (0.79-1.02) |
| Quintile 4 | 434 | 0.85 (0.75-0.98) |
| Quintile 5 | 398 | 0.81 (0.71-0.93) |
| P for trend | <.01 | |
| Walking (MET-hr/wk)‡ | ||
| Quintile 1 | 524 | Referent |
| Quintile 2 | 524 | 1.01 (0.88-1.14) |
| Quintile 3 | 470 | 0.91 (0.80-1.04) |
| Quintile 4 | 463 | 0.90 (0.78-1.04) |
| Quintile 5 | 374 | 0.74 (0.63-0.88) |
| P for trend | <.01 | |
MET, metabolic equivalent task; OR, odds ratio; CI, confidence interval.
* Incontinence cases were defined as leaking urine at least once per week.
† Odds ratios were adjusted for age, race or ethnicity, body mass index, parity, cigarette smoking, and postmenopausal hormone therapy.
‡ Walking analyses were controlled for total activity.
From Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol 2007;109(3):721–7.
The ICI committee concluded that “strenuous exercise is likely to unmask the symptoms of SUI during the provocation. There is currently no evidence that strenuous activity causes the condition of UI” and “there is good prospective cohort information suggesting that moderate exercise decreases the incidence of UI in middle aged and older women” (level of evidence II) (Hay-Smith et al, 2009).
Other Health Issues
Increasing attention has been paid to the relationship between diabetes and UI. Data from the Nurses’ Health Study and the Nurses’ Health Study II were examined to study associations between diabetes and UI type in 71,650 women (Danforth et al, 2009). Incident UI cases were identified over a 2-year period. The incidence of at least weekly UI was 5.3% among women without type 2 diabetes and 8.7% among women with diabetes (adjusted odds ratio 1.2; 95% CI 1.0 to 1.3; P = .01). This increase appeared largely explained by significantly greater odds of UUI (OR 1.4; 95% CI 1.0 to 1.9; P = .03). A cross-sectional mail survey of community dwelling women screened women for pelvic floor disorders while diabetes status and other risk factors were obtained from medical record review. Of 3,962 women, 393 (10%) had diabetes. Among women with diabetes, being obese was associated with SUI and OAB (Lawrence et al, 2007). This finding was supported by analysis of an RCT comparing treatment of diabetics with UI using oral hypoglycemics (metformin) versus lifestyle intervention (weight loss and exercise) (Brown et al, 2006). The researchers found that the prevalence of UI was lower in women randomized to lifestyle intervention than those on medication or placebo.
There is a strong relationship between UI and depression. In one study, patients with UI were almost three times more likely to have major depression than those without (6.1% vs. 2.2%) and patients with UI and depression had significantly greater decrements in quality of life and functional status than those with UI alone (Melville et al, 2005a).
A specific investigation into comorbidities and UI was undertaken by a research group in the United Kingdom who note, “The bladder forms part of a sensitive and complex system of the body that operates largely autonomously but remains under voluntary control. As such, it is vulnerable to general disease processes related to ageing. Thus, associations with poor health and obesity could represent pathogenic as well as functional involvement of the bladder.” (McGrother et al, 2006). They used a postal survey of a random sampling of over 19,000 community dwelling women older than 40 years of age with 1-year follow-up surveys. There were strong correlations between poorer health and increased prevalence of both SUI and OAB. There are many interesting findings but one of the most important is that “OAB was independently predicted by poor health … The association with old age, although consistent with other studies, disappeared after controlling for a full range of specific comorbidities, suggesting that the condition is age related rather than age dependent.”
Key Points: The Tools of Conservative Therapy: Behavioral Therapy
Concept of “Therapeutic Package”
Such an approach showed utility in an RCT in which patients were randomized to receive a package of behavioral therapy including individualized counseling about fluid and caffeine intake, quick pelvic floor muscle contraction, voiding frequency, and management of constipation or placed on a waiting list (Kincade et al, 2007). This design provides a good starting point for use in many patients with UI. In addition, Diokno and colleagues (2004b) demonstrated that two simple group sessions were effective in preventing UI. The trial randomized 359 continent women older than age 55 years who were recruited and observed for 1 year. The treatment group received a single 2-hour group session teaching pelvic floor muscle training (PFMT) and bladder training as well as an audiotape on PFMT for reinforcement; the control group was not treated. There was one follow-up office visit with a nurse specialist in 2 to 4 weeks. At the end of 1 year, 56% of the treatment group reported the same or better continence compared with only 41% of controls; 37% of the treatment group reported “absolute continence” compared with 28% of the controls. Several other studies using various combinations of therapies led the ICI group (Wilson et al, 2005) to a level A recommendation that “women with stress, urge, or mixed incontinence should be offered a conservative management program as first line therapy for UI” while acknowledging that there were inadequate data to define the optimal treatment package and that most of the data comes from trials in older women.
Pelvic Floor Rehabilitation
Pelvic Floor Muscle Training
Pelvic floor exercises (PFE) or “Kegels” have been advocated in the treatment of UI since the 1950s (Kegel 1948a, 1948b, 1956). Dr. Kegel taught his patients to forcefully contract the pelvic muscles to treat and prevent postpartum UI. Unfortunately, half of patients are unable to perform a proper contraction with simple instructions (Bump et al, 1991), and up to one fourth will actually promote UI with their efforts. Indiscriminant recommendation of this therapy to all incontinent patients has created a negative bias that PFE are just “something to do” before pharmacologic or definitive surgical treatment. It should also be acknowledged that Dr. Kegel routinely employed a perineometer with his patients in one of the first documented uses of biofeedback in medicine. This implies that Kegel exercises alone should not be considered the standard treatment for pelvic floor rehabilitation.
Currently, PFMT is the currently accepted term, replacing Kegels and PFE. It is defined as “any program of repeated voluntary pelvic floor muscle contractions taught by a health care professional” (Wilson et al, 2005) and is advocated for both prevention and treatment of UI. “Training” is preferred over “exercises” to emphasize the importance of a regimen of repeated exercise over time. The therapy is intended to improve the function of the pelvic floor muscles; whether this happens primarily by increasing the strength, power, and speed and/or improving the timing and coordination of a contraction is not known. Much more is known about the clinical effectiveness of the therapy than the exact physiologic changes that produce the outcomes. With repeated exercise a muscle will develop improved responsiveness that may lead to a faster and/or stronger contraction before any increase in actual bulk. Over longer periods of time the muscle fibers will progressively hypertrophy, producing increased bulk. The ICI committee (Hay-Smith et al, 2009) points out that (1) an increase in strength occurs before visible hypertrophy, (2) early improved strength results from neural adaptation, and (3) hypertrophy begins only after a minimum of 8 weeks and may continue for some years. In any case, muscle bulk is clearly not a prerequisite for improved continence with PFMT, and many studies have failed to show a correlation between improved strength and improved continence.
When possible these issues are addressed in the following discussion. The interested reader is strongly encouraged to study the comprehensive review performed by the ICI committee (Hay-Smith et al, 2009).
Does PFMT Prevent Urinary Incontinence in Childbearing Women?
PFMT has been used to prevent UI in pregnant women, because pregnancy and vaginal birth are strong risk factors for UI. Three prospective RCTs have investigated this question (Sampselle et al, 1998; Reilly et al, 2002; Mørkved et al, 2003), producing a grade A recommendation that primiparous women “should be offered a supervised and intensive strengthening antepartum PFMT programme to prevent post-partum UI” (Hay-Smith et al, 2009). In all three trials the PFMT group had less postpartum UI; and in one study (Reilly et al, 2002), the effect was still present at 4 years postpartum, although only 100 of 268 patients were available for follow-up. There was no evidence supporting the utility of PFMT in pregnant, previously continent, multiparous women. The evidence reviewed by the ICI group for postpartum PFMT was less compelling, but it was concluded that the evidence also supports a grade C recommendation for supervised intensive PFMT for women after delivery utilizing instruments or delivery of a large infant (≥4000 g) (Hay-Smith et al, 2009).
Is PFMT an Effective Treatment for Urinary Incontinence and Which Patients Are Good Candidates?
The most important question is whether PFMT is effective in the management of established UI and, if so, how to most effectively apply the therapy to the large population of incontinent women. Seventeen RCTs comparing PFMT to no intervention, placebo, sham, or control in childbearing women were summarized by Hay-Smith and colleagues (2009) and are based on the findings of a Cochrane review (Dumoulin et al, 2008). Although the magnitude and duration of the expected effect is not well defined, the overwhelming majority of the trials showed effectiveness of PFMT, leading to a grade A recommendation that “PFMT should be offered, as first line therapy, to all women with stress, urge or mixed incontinence.” This recommendation is, of course, based on the combination of demonstrable effectiveness with essentially no risk to the patient. It does not mandate that all patients undertake a formal course of PFMT before surgical treatment. However, there are as yet no studies that adequately define patient groups who are unlikely to respond to PFMT based on clinical factors such as age, obesity, and so on. In most cases, studies have been too small to examine such predictive factors and there may well be a role for individualized therapeutic recommendations if proper studies could be done. A single trial investigating PFMT for UI in pregnancy did not show efficacy (Woldringh et al, 2007), but this has been criticized owing to lack of specificity about the intervention. The ICI committee specifically points out the need for a large scale, pragmatic trial of PFMT with detailed reporting of long-term (>5 years) follow-up (Hay-Smith et al, 2009). The authors also describe what they believe are the key components of an effective program: (1) it is based on sound principles (specificity, overload, and progression), (2) correct contraction is confirmed before training, and (3) women are supported to maintain treatment adherence (level of evidence 4).
The quality of the data for men with postprostatectomy UI is less robust with too many single-center, underpowered trials. Research has focused on use of pelvic floor therapies to hasten return of continence. A systematic review of the literature concluded that although training may hasten return of continence after surgery it does not affect long-term outcome (MacDonald et al, 2007). Others have found that there is no difference between routine preoperative and postoperative PFMT (Filocamo et al, 2005). In contrast, one RCT found that significantly more men were completely continent 1 year after prostatectomy when performing PFMT with a physiotherapist than without (Overgard et al, 2008). When PFMT is used for established postprostatectomy UI, the results similarly do not establish long-term benefit of therapy, with or without biofeedback. Interested readers are referred to a Cochrane review on the subject (Hunter et al, 2004).
Within these overarching categories it is likely that specific patient subgroups are more or less likely to benefit from PFMT (and probably other conservative techniques). However, studies have not been designed to specifically identify such groups and subgroup analysis of relatively small trials is fraught with error. In one retrospective series of 447 women with SUI, 49% of patients were “successfully treated.” Success appeared to be more likely in patients with milder degrees of UI (not using pads: 67% success; not having daily UI: 63% success; no leakage at first cough: 60% success). Independent risk factors for failure included two or more leaks per day, presence of leakage at first cough, and use of antidepressant/anxiolytic medications (Cammu et al, 2004). Burgio (2004)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree