Craig A. Peters, MD, FACS, FAAP, Robert L. Chevalier, MD The spectrum of urinary obstruction in children is one of the most common conditions affecting the urinary tract and has substantial health consequences. Obstructive nephrouropathies constitute the single largest entity leading to renal insufficiency in male children younger than 1 year of age and are the largest single cause of renal failure requiring transplantation, occurring in about 23% of children who undergo renal transplantation (Seikaly et al, 2003). A large number of children are affected by lesser degrees of obstruction that induces concern, leading to ongoing imaging to monitor renal function and the status of the obstruction. The wide spectrum of obstructive changes constitutes one of the major challenges in the clinical management of these conditions in that there is no definitive dividing line between obstruction that warrants intervention from that which does not (Peters, 1995). The presence of an obstructive lesion is readily determined with current imaging, but the criteria for intervention are highly controversial. This is largely because of a lack of information regarding the natural history of differing degrees of obstruction and the absence of effective markers of the pattern and progression of obstruction (Chevalier, 2004). We are left with few guideposts along the spectrum of obstruction by which clinical decisions may be made. Prenatal ultrasonographic diagnosis has radically altered the clinical presentation of obstructive conditions, and today most of these conditions are detected before birth and many have no apparent clinical signs. Those that present as clinical manifestations are often severe obstructive conditions, such as posterior urethral valves or massive hydronephrosis. Children are still found with clinical signs of obstruction, usually infection or pain and, rarely, hypertension. In the child with obstruction of the entire urinary tract, even when the obstruction is relieved the functional abnormalities inform urologists of the effects of obstruction on developing kidneys. Reduced filtration function as manifest by a rising serum creatinine concentration is associated with glomerular injury, acidosis is due to tubular injury, and nephrogenic diabetes insipidus is secondary to collecting duct abnormalities. In extreme cases these may all be present, but on occasion they are noted in isolation and will often persist after correction of the obstruction (Hutcheon et al, 1976). Obstructive hypertension appears to be renin mediated (Riehle and Vaughan, 1981; Urata et al, 1985; Mizuiri et al, 1992) and may be reversible with surgical repair (de Waard et al, 2008). The pathologic correlates of these functional alterations have been described in the congenitally obstructed kidney to varying degrees (Elder et al, 1995; Stock et al, 1995; Han et al, 1998; Poucell-Hatton et al, 2000; Zhang et al, 2000; Huang et al, 2006). The pathologic changes associated with lesser degrees of obstruction have been less thoroughly investigated, and a spectrum of qualitatively similar alterations have been described. In the absence of overt functional alterations and in unilateral conditions, determining the state of the affected kidney becomes a clinical challenge. This challenge is often tied to the question of whether surgical intervention is appropriate, and much controversy has emerged from this question. Clinical imaging studies are currently the only widely used modality to make this assessment, and their interpretation is not uniform. The natural history of many conditions in the spectrum of obstruction is not well described yet is the essence of the question. It should be clearly seen that the spectrum of obstruction is wide, involves conditions that do not require intervention as well as others that produce profound renal injury, and may change with age and persistence of the condition. A major concern regarding obstructive conditions in the urinary tract is the potential for a progressive situation leading to ever more loss of renal function. Progression may be seen in two forms, one with the uncorrected partially obstructive lesion (i.e., ureteropelvic [UPJ] obstruction) and the second with the previously obstructed but corrected obstruction that has produced some degree of renal damage (i.e., posterior urethral valves). In the first, renal function may initially appear intact on imaging tests yet in time there will be progressive loss of absolute and relative function of the kidney affected by the obstruction. If this were known prospectively, intervention would be appropriate and should be performed early. The challenge is in predicting this situation, and few markers are available to do this. The frequency of progressive deterioration in unilateral obstruction likely ranges from 20% to 40%, but duration of follow-up will significantly affect this rate. Every prospective study of obstructive conditions has documented the potential for progressive loss (Palmer et al, 1998; Parkhouse et al, 1988; Koff and Campbell, 1992; Koff, 2000). The second situation reflects the fact that the damaged kidney does not have the functional reserve of a normal kidney by which it may maintain its absolute function over time. These kidneys will demonstrate a steady decline in function over time, and this is usually evident in bilateral obstruction. The mechanism may be hyperfiltration of remnant renal units, and clinically this is most often seen with posterior urethral valves (Parkhouse et al, 1988; Nguyen and Peters, 1999). These children may show very adequate renal function early in life yet demonstrate a delayed and inexorable progression into renal failure in adolescence (Fig. 113–1). Whether earlier intervention would have protected their renal function is uncertain, but that possibility cannot be neglected. (From Roth KS, Carter WH Jr, Chan JCM. Obstructive nephropathy in children: long-term progression after relief of posterior urethral valve. Pediatrics 2001;107:1004–10.) Although it may not be difficult to diagnose the potentially obstructed urinary tract, usually by ultrasonographic identification of hydronephrosis, the determination of whether this particular condition requires surgical intervention to protect renal function and development is much more difficult. The frequently quoted definition of congenital obstruction (Koff, 1987), which is “a condition producing a restriction of urinary flow that will lead to deterioration in renal function,” is too limited. In the growing child, renal function is expected to increase markedly, initially in excess of body mass and subsequently in parallel to it. If this increase in function does not occur, renal functional potential is lost, which may be of greater consequence than losing absolute function. Therefore the impairment of renal functional development should be considered as a determinant of obstruction that warrants intervention (Peters, 1995). A critical element of congenital urinary obstruction is the recognition that it is distinct from acquired obstruction in the mature animal (or mature kidney). The fact that obstruction develops at the same time the kidney is in the process of formation creates an entirely different paradigm for congenital urinary obstruction as compared with obstruction of the mature kidney (Peters, 1997). The patterns of renal development are affected by obstruction, and this produces the ultimate functional effects. The mechanisms for these alterations are therefore wholly distinct from mature obstruction. Although there is likely to be some overlap in the critical mechanisms of change, many will be different. It cannot be assumed that the principles relevant to mature obstruction are applicable to congenital obstruction. This will clearly impact the interpretation of experimental data in the postnatal and mature animal, as well as the selection and application of experimental systems. In an attempt to understand the pathophysiology of congenital urinary obstruction, examination of obstructed renal tissues has shown disorganization of structure with primitive forms of renal tissues—dysplasia. Although the definition of dysplasia is not universally agreed on, evidence of altered renal development in association with obstruction is frequently reported (Bernstein, 1971; Matsell, 1998; Tarantal et al, 2001; Matsell et al, 2002; Matsell and Tarantal, 2002). Dysplasia is often considered to be an embryonic process and irreversible, yet this is not proven. It is clear that some dysplasias are due to very early abnormalities of renal development (Winyard and Chitty, 2008), unrelated to obstruction, but it is equally clear that obstructive processes can produce dysplasia. Whether there are subtle differences between these patterns is unclear, but it should not be difficult to understand that several underlying causes may produce similar outcomes when renal developmental patterns are disrupted. In some cases, however, usually in lesser degrees of obstruction, there is no dysplasia (Bonsib, 1998; Zhang et al, 2000). In such cases the mechanisms of obstructive effect may be similar but to a lesser degree or they may be entirely different. It is important to recognize the variability of the patterns of response to obstruction. Structural alterations with obstruction are obviously evident with hydronephrosis, but this is largely a distortion of normal architecture and relative alterations in the amounts of elements of renal tissues. There may be subtle changes, however, that are functionally important. Other alterations include fibrosis and increased interstitial tissues, as well as the presence of abnormal tubules and glomeruli. The normal layered organization of the kidney with the cortical and medullary areas with inner and outer stripes is often distorted or absent (Zhang et al, 2000). Less obvious will be changes in the differentiation of the individual renal cell types that make up the nephron (Huang et al, 2006). Cells are unlikely to perform their normal functions, including communicating with neighboring cells in an integrated fashion. Function will therefore be disrupted. These observations are supported by both clinical and experimental work. Several biopsy studies have shown patterns indicative of altered differentiation and growth in various levels of obstruction, including both upper (UPJ obstruction) (Elder et al, 1995; Stock et al, 1995; Zhang et al, 2000; Huang et al, 2006) and lower (posterior urethral) valves (Poucell-Hatton et al, 2000; Haecker et al, 2002). These variations are not well explained, and correlation with clinical parameters is often imperfect. Experimental studies, however, have shown similarly that obstruction during development will produce changes in patterns of renal differentiation and in growth regulation (Beck, 1971; Steinhardt et al, 1988; Gonzalez et al, 1990; Peters et al, 1992; Wen et al, 2002; Cachat et al, 2003; Mure et al, 2006b). These can be seen to vary depending on the time of onset in experimental models, as well as the severity of obstruction (Fig. 113–2) (Chevalier et al, 1988, 1999c; Thornhill et al, 2005). The latter is difficult to measure accurately. The validity of any of these model systems must be challenged, however, until we have more definite correlations. Nonetheless it is clear that induced obstruction can produce such severe abnormalities of differentiation as to be considered dysplasia and to produce clear disruptions of growth regulation (Peters et al, 1992). It is therefore reasonable to examine the specific patterns observed and the potential mechanisms of those changes, all of which are likely contributing factors in the development of obstructive nephropathy. (Modified from Thornhill BA, Burt LE, Chen C, et al. Variable chronic partial ureteral obstruction in the neonatal rat: a new model of ureteropelvic junction obstruction. Kidney Int 2005;67:42–52.) The effects of obstruction on the developing kidney may be summarized as producing alterations in the regulation of growth, tissue differentiation, extracellular matrix (ECM), and fibrosis and in altering the functional integration of the kidney (Fig. 113–3). The latter is largely a result of the first three major factors and refers to the mechanisms producing vascular, neural, and humoral homeostasis and in the regulation of inflammatory cascades. Understanding the mechanisms by which these systems are dysregulated by obstruction will permit a better understanding of the outcomes of obstruction, which should improve our diagnostic, prognostic, and therapeutic abilities. Figure 113–3 Diagram of the key patterns of effect due to obstruction during fetal life. UPJ, ureteropelvic junction. Key Points: Patterns of Congenital Obstructive Nephropathy Growth regulation is a critical part of development, and the obstructed kidney may evidence impaired or accelerated growth. It is important to recognize that the small, obstructed kidney is not atrophic, as might be seen in the adult, but is hypoplastic. The growth it should have experienced never occurred. This can be readily seen on prenatal ultrasound evaluation and has been shown repeatedly experimentally (Peters et al, 1992; Mandell et al, 1994). This seems usually to be a generalized impairment of all parts of the kidney, with both reduced numbers of nephrons as well as smaller nephrons. Differential growth impairment within the nephron segments may be present as well (Cachat et al, 2003; Huang et al, 2006). The functional effects of significant growth impairment are obvious because there are fewer and smaller nephron units. There may be compensatory responses to these changes, and it is difficult to assess their long-term impact. Reduced renal mass can be associated with hypertension as well as reduced filtration function, and loss of tubular mass will affect electrolyte and acid-base homeostasis, as well as water balance. Growth acceleration can be seen in the larger than normal hydronephrotic kidney, although this is difficult to prove in humans, because few of those kidneys are removed. In animal experiments, fetal partial obstruction can increase renal mass (Gobet et al, 1999a; Ayan et al, 2001). This is not seen with dysplastic changes and is not a product of edema, because the total protein and DNA are increased as well. The factors that predict altered growth appear to be the severity of obstruction and timing of the obstructive effect, although the latter is difficult to ascertain in human situations. The functional consequences of accelerated growth are not known, and this may correlate with the occasional hyperfunction measured on renal scans that is seen in some patients with hydronephrosis (Moon et al, 2003; Maenhout et al, 2005). Compensatory effects with obstruction may not be benign; growth enhancement of the glomeruli occurs in early diabetes and is later associated with glomerular sclerosis. Regulation of renal growth is extremely complex and dynamic through development. A variety of growth factors are known to influence kidney growth at various stages of renal development and act at different loci of the nephron. Obstructive conditions have been shown to alter expression of growth-regulatory genes as well as the presence of the proteins coded by these genes (Chevalier, 1996). Some of the factors known to be altered in renal obstruction (not all in early or fetal models) are listed in Table 113–1. Epidermal growth factor (EGF) has been shown to reduce some of the growth effects of obstruction when administered exogenously (Kennedy et al, 1997; Chevalier et al, 1998, 1999a). Many studies have focused on specific growth factors, but it is evident from the complexity of renal development and growth regulation that the interactions of multiple factors and their signaling pathways will be of greater relevance. A critical component of growth in the developing kidney is apoptosis—regulated cell death. The early fetal kidney is extremely active in terms of new cell formation as well as turnover (Carr et al, 1995). This permits remodeling during development, as well as providing a control system over unregulated growth. Small increases in the rate of apoptosis, even with normal ongoing growth, would lead to significant reductions in renal mass over time. The role of apoptosis in congenital obstruction has become more firmly established in recent years, including cellular patterns characteristic of apoptosis and enhanced expression of apoptosis-regulating molecules (Yang et al, 2001; Yoo et al, 2006; Eskild-Jensen et al, 2007a; Campbell et al, 2008) (see Table 113–1). The changes may be seen heterogeneously, and the precise means by which these alterations occur remain incompletely defined, although apoptotic activity is regulated by cytokines (Cohen et al, 2007; Campbell et al, 2008) as well as mechanical factors (Nguyen et al, 2000; Hsieh and Nguyen, 2005). Inappropriate apoptosis may also be related to interstitial fibrosis as well (Docherty et al, 2006a). An important aspect of understanding the role of apoptosis in congenital obstruction is that the mediators may be measurable in the urine or blood, and they may permit therapeutic manipulation (Mizuguchi et al, 2008). Differentiation is the process of cells attaining specific functional traits to permit specialized functions and organization into tissues. It is the basis for renal function, in its many aspects. Obstruction affects these finely tuned patterns, as can be seen histologically in a severely obstructed kidney with dysplasia. More subtle effects may require assessment of tubular or glomerular function, but all are a result of altered differentiation. Disruption of differentiation may begin as early as induction of the nephron or later in development with injury to renal collecting duct cells that regulate urinary concentrating ability. Some of these changes may be reversible, but it is usually presumed that many are not, in that some cells will undergo terminal differentiation, and if that does not occur at a particular time point in development there will not be another opportunity for it to occur. Disruption of normal differentiation of renal elements is unique to congenital obstruction and does not occur in a major way in adult obstruction. Abnormal epithelial-mesenchymal transformation (EMT) is one alteration in differentiation that does occur in the adult and can be reversible (Hay and Zuk, 1995; Yang et al, 2005; Docherty et al, 2006b; Forino et al, 2006; Higgins et al, 2007; Ivanova et al, 2008). A variety of mediators of EMT have been described, including transforming growth factor-β (TGF-β), plasminogen (Zhang et al, 2007), and leukocytes (Lange-Sperandio et al, 2007), as well as inhibitors, such as hepatocyte growth factor (HGF) (Yang et al, 2005) (Fig. 113–4). It is likely to be an important factor in fetal obstruction as well, although little is known about its role. Most adult cells may be injured and lose their differentiated capacity, but the cell types rarely change their behavior once differentiated. Understanding the patterns of altered differentiation and its regulators is critical to understanding congenital obstructive nephropathy. Renal differentiation begins with induction and from then on is exquisitely sensitive to various outside effects that may disrupt the normal sequence of cellular changes that results in a kidney. When these may be disturbed by obstruction is unclear, but it can be presumed that some of the variation in the spectrum of obstruction is due to different times of onset of the obstructive effect and to different degrees of severity. The pattern of effect is likely to reflect the time of onset and be reflected in the number of nephron generations and the degree of dysplastic transformation. Controversy remains as to whether obstruction can produce dysplasia, and it is often stated that if dysplasia is present, then it was due to abnormal induction and not obstruction. Abnormal induction and nephrogenesis may be produced by various factors, including genetic ones, or due to cellular disruption from mechanical forces with subsequent cellular responses. The study of Maizels and Berman is often cited to indicate that dysplasia in the chick kidney (a mesonephric kidney) was only produced with mechanical disruption of the mesenchyme and not hydronephrosis. The production of “obstruction” in that elegant study was by necessity rather crude, and several of the preparations were not obstructed. It is unclear whether they all were to a sufficient degree. It also suggests that mechanical forces can, in fact, disrupt nephronogenesis enough to produce dysplasia, and there is no inherent reason to believe that this cannot be from obstruction as well. Later mammalian studies in fetal sheep have shown dysplastic changes produced by obstruction (Steinhardt et al, 1988; Peters et al, 1992; Matsell et al, 1996), and this has also been shown in rodent studies (Thomasson et al, 1970). The critical determinant of dysplasia in animal studies has been complete obstruction early in gestation. In the fetal sheep this was only seen when the obstruction was induced before 50% of gestation (70 days in most sheep species, which have a gestation from 140 to 145 days). Obstruction induced after that point only produces hydronephrotic changes, albeit severe ones (Beck, 1971). Partial obstructions produced hydronephrosis only, without apparent disruption of the renal architecture. The reason for this is presumably altered sensitivity of the developing nephrons to obstructive effects at this point. Alternatively, the particular signaling systems that are active in the early phases of renal development begin to fade away with ongoing development. It is possible that the pathways sensitive to obstruction that would inherently alter the pattern of development have run their course of expression and activity by mid gestation. Expression of recognized mediators of renal development have been shown to be affected in models of fetal obstruction, including WT1 (Liapis, 2003), the Wnt gene family (Nguyen et al, 1999), and PAX2 (Attar et al, 1998; Mure et al, 2006b; Cohen et al, 2007). One of the histologic hallmarks of renal dysplasia is fibromuscular collars surrounding tubular structures, so-called primitive ducts. These have a characteristic appearance, and the smooth muscle surrounding the tubules stains for α-smooth muscle actin (α-SMA) (Fig. 113–5). This pattern suggests an abnormality in the regulation of EMT (Butt et al, 2007; Baum et al, 2008). The mesenchymal structures of the primitive nephrogenic blastema and the epithelium of the ureteral bud processes interact, and there is differentiation from an epithelial to mesenchymal phenotypes and the reverse. It is uncertain whether the presence of the primitive tubules suggests persistence of mesenchyme that should have transformed to epithelium or inappropriate EMT. Understanding the signaling pathways involved in these processes will directly impact our understanding of obstructive processes (Roberts et al, 2006; Bani-Hani et al, 2008). Persistent expression of α-SMA can be seen in partial obstruction without dysplastic patterns and thus may be important at several levels of severity (Gobet et al, 1999; Mure et al, 2006a). α-SMA expression is regulated by TGF-β1, linking these two proteins to growth as well as to fibrotic changes. (From Gobet R, Bleakley J, Cisek L, et al. Fetal partial urethral obstruction causes renal fibrosis and is associated with proteolytic imbalance. J Urol 1999;162:854–60.) Development of the glomerulus is a tightly regulated process that involves interaction of the mesenchyme and epithelium with a very specific pattern of growth and formation of intermediate structures such as the S-shaped body. In general, primitive glomeruli are not evident in obstructive changes but markedly abnormal glomeruli are seen as well as hypoplastic glomerular structures (Matsell et al, 2002). In some cases, glomeruli may be enlarged in obstruction, suggestive of the changes seen in hyperfiltration that lead to glomerulosclerosis. Dissociation of the glomerulus from the tubules can be seen in early neonatal rodent obstruction (Thornhill et al, 2007), suggesting severe disruption of the developmental program. Key Points: Growth and Differentiation A universal characteristic of obstructive nephropathy appears to be renal fibrosis, although it is a nonspecific pattern seen in a variety of pathologic conditions affecting the kidney (Eddy, 2000). It is seen as infiltration of the interstitium with abnormal amounts of ECM, including collagens, fibronectin, and other connective tissue proteins. Their presence disrupts the normal interconnections between cells that permit functional integration of the renal tissues. Tubular cells may be disrupted and inadequately regulated. Cell–cell signaling by direct connection or paracrine messengers may be disrupted. Tissue oxygenation may be impaired. Fibrosis is a histopathologic hallmark of many renal diseases. The ECM is essential to normal function of the kidney as well, in terms of providing structural integrity and contributing to normal signaling systems. When abnormally expressed, however, it becomes detrimental. ECM homeostasis is a complex balance of synthesis and breakdown. Synthetic regulation is controlled by various mechanisms, including growth factors and signaling systems that are just being discerned. Mechanical forces contribute to these signals in various conditions, including hypertension and hydronephrosis. ECM breakdown is tightly regulated and represents the product of degradative enzymes, the matrix metalloproteinases (MMPs), and their endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). This product is the proteolytic balance and is regulated by various cytokines, hormones, and mechanical forces. This balance has been studied vigorously in renal disease and to a limited degree in congenital obstruction (Engelmyer et al, 1995; Ayan et al, 2001; Mure et al, 2006a). The interaction of these systems is complex, and various compensatory pathways are likely to be present (Kim et al, 2001). Modulation of renal fibrosis may be a significant potential target for managing obstructive nephropathy, but the delicate balance of these factors needs to be understood to a greater degree than at present (Eddy, 2005). Increased interstitial connective tissue is a hallmark of various renal pathologic processes (Eddy, 1996), including obstruction. Although it is unclear if the mechanisms of fibrotic change are universal, they are believed to interfere with intercellular signaling and thus with functional integration (Fig. 113–6). The most likely causes of excessive connective tissue include abnormal accumulation due to imbalance of synthesis and breakdown. It may also represent abnormal inductive signaling that produces excessive conversion of epithelium to mesenchymal tissue and connective tissues (Bascands and Schanstra, 2005; Burns et al, 2007; Zhang et al, 2007). These processes may be normal developmental sequences that persist due to the obstructive effect. Abnormal accumulation may represent simply excessive synthesis with no change in ECM breakdown. Increased collagen synthesis has been shown by upregulation of collagen gene expression in obstructive models (Liapis et al, 1994; Fu et al, 2006). Collagen synthesis is regulated in part by various cytokines, including TGF-β and the renin-angiotensin system (RAS). Reduced fibrosis may be seen when angiotensinogen activity is downregulated in an obstructed system (Fern et al, 1999; Kellner et al, 2006). Similar approaches have been demonstrated in nonobstructive fibrosis as well. The role of TGF-β is complex and context dependent, yet inhibition of activity will produce less fibrosis as well (Isaka et al, 2000; Miyajima et al, 2000; El Chaar et al, 2007). There is evidence that TGF-β interacts and regulates the activity of the RAS, as well as regulating cellular growth dynamics. Fetal obstruction induces increased expression of TGF-β1 (Medjebeur et al, 1997; Ayan et al, 2001). As reviewed earlier, TGF-β1 regulates EMT, thereby contributing to renal dysplasia (Yang et al, 2000). There is considerable interest in the role of TGF-β1 in promoting EMT of differentiated renal tubular epithelial cells in response to chronic ureteral obstruction. As shown in Figure 113–4, renal interstitial fibroblasts, through transformation to myofibroblasts, play a pivotal role in the deposition of ECM in a hydronephrotic kidney. There is growing evidence that, in addition to proliferation of resident interstitial fibroblasts and transformation of hematopoietic stem cells, renal tubular epithelial cells can undergo EMT and migrate to the interstitium to contribute to the pool of fibroblasts (Iwano et al, 2002). Many of the steps involved in the transformation of tubular epithelial cells are regulated by TGF-β1; these steps include loss of cell adhesion, expression of α-SMA, disruption of tubular basement membrane, and cell migration to the interstitium (Liu, 2004). Signaling by TGF-β1 is mediated in part by Smads, which are both profibrotic (Smad2 and Smad3) and inhibitory (Smad7). Thus targeted deletion of Smad3 reduces apoptosis and fibrosis in mice with ureteral obstruction, whereas gene therapy with Smad7 also reduces fibrosis (Lan et al, 2003; Sato et al, 2003). TGF-β1 also acts through downregulation of transcriptional corepressors of the Smads: SnoN and Ski (Yang et al, 2003b). In contrast, HGF blocks EMT by blocking Smad2 and Smad3 and gene therapy with HGF reduces fibrosis in rats with ureteral obstruction (Gao et al, 2002; Yang and Liu, 2003). HGF also acts by upregulating the Smad corepressors SnoN and Ski, countering the action of TGF-β1 (Yang et al, 2005). These complex networks of counterbalancing factors provide many potential opportunities for therapeutic intervention to prevent the progression—or even promote the reversal—of interstitial fibrosis resulting from obstructive nephropathy (Fogo, 2003). Nitric oxide has also been shown to regulate the development of obstructive fibrosis in postnatal animals and may play a similar role prenatally (Huang et al, 2000; Felsen et al, 2003). Increased nitric oxide generation reduces the degree of interstitial fibrosis (Ito et al, 2004), and in animals without the gene to produce nitric oxide synthase, unilateral obstruction produces a greater degree of fibrosis than in those animals with this enzyme intact (Hochberg et al, 2000). Data suggest a greater potential role for nitric oxide derived from endothelial nitric oxide synthase (eNOS) than inducible nitric oxide synthase (iNOS) (Huang et al, 2000; Chang et al, 2002). Hypoxia may be a factor in the development of tissue fibrosis as well, in response to induction of hypoxia-inducible factors-1 and 2 (HIF-1, HIF-2) (Higgins et al, 2008). In-vitro stimulation of renal epithelia with HIF-1 increases EMT, known to induce fibrosis. Genetic models that do not express HIF-1α develop less fibrosis and inflammatory infiltration in response to ureteral obstruction in postnatal models (Higgins et al, 2007). The role in prenatal obstruction remains undefined but potentially relevant. Altered regulation of ECM breakdown, the proteolytic balance, is a potential mechanism for interstitial fibrosis of obstruction as well, although less explored. Connective tissue breakdown is controlled by the MMPs, of which there are at least 15 having specific activity toward particular connective tissue proteins. MMP-1, for instance, is a collagenase (Pardo and Selman, 2005), whereas MMP-2 specifically degrades gelatin. Their expression and activity are both subject to close regulation, because it is crucial to appropriate ECM homeostasis. Too much activity will degrade the tissues, whereas too little permits accumulation of abnormal amounts of ECM. These pathologic alterations have been described in a variety of disease states, including arthritis and pulmonary fibrosis (Corbel et al, 2002; Vincenti and Brinckerhoff, 2002). One of the means by which their activity is regulated is through endogenous TIMPs. These are less varied and may have less specific activity over the MMPs in their environment and serve to check the degradative activity of the MMPs. Their expression and activity are closely regulated and their net activity is the proteolytic balance. The role of the proteolytic balance in a wide variety of disease states has been the subject of active research (Diamond et al, 1998; Vincenti, 2001). Altered activity and regulation of the MMPs and TIMPs have been studied in the kidney (Eddy, 1996) in several conditions, and it is clear that they are important regulators of the state of the ECM. Their expression has been shown in the developing kidney as well, and in postnatal obstruction they are altered in activity. In prenatal obstruction increased expression (Ayan et al, 2001; Mure et al, 2006a) and activity (Gobet et al, 1999a) have been shown, as well as in congenital reflux-related fibrosis (Gobet et al, 1998). The precise regulators of the MMPs and TIMPs in development and obstruction are not well defined but are likely to be an important element in the development of pathologic fibrosis. There also is some evidence of regulation by the RAS and TGF-β, which opens therapeutic options for the treatment of fibrosis (Diamond et al, 1998; Ding et al, 2005; Bolbrinker et al, 2006; Yang et al, 2007). Inflammation appears to be a common consequence of obstruction in the postnatal human or animal model. Much attention has been paid to this in acquired obstruction, but it is surprisingly absent in congenital obstruction. Biopsies of human kidneys with obstruction but no history of infection have sparse inflammatory infiltrates (Huang et al, 2006). Models of fetal obstruction have little evidence of inflammation (Peters et al, 1992). The reason for this marked difference is unclear but suggests fundamentally distinct mechanisms. It is another indication that congenital obstruction is distinct in many ways from postnatal acquired obstruction. Expression of renal renin is increased in the obstructed kidney (el-Dahr et al, 1993; Gobet et al, 1999b; Ayan et al, 2001) and decreased in the contralateral kidney. Renin-secreting renal cortical cells appear to be recruited with obstruction as well (Norwood et al, 1994). Expression of receptors for the RAS is altered in specific patterns in neonatal obstruction. Angiotensin I, which mediates vasoconstriction (as well as growth alterations) (Chung et al, 1995; Yoo et al, 1998) increases whereas the levels of angiotensin II, which acts in a contrary manner, are decreased. These alterations suggest a role for a local renal RAS in obstruction-mediated vasoconstriction and that are independent of the systemic RAS. Altered sensitivity of both kidneys to renin-mediated vasoconstriction has been shown to occur with unilateral obstruction. Interaction with nitric oxide has been seen to be important in regulation of both glomerular and tubular function in early obstruction (Eskild-Jensen et al, 2007b). After release of obstruction, sensitivity to angiotensin II–mediated vasoconstriction was reduced in the obstructed kidney but increased in the contralateral kidney (Chevalier and Gomez, 1988). These observations indicate the sensitivity of renal vascular regulation to obstruction as well as the importance of recognizing the interaction of the two developing kidneys. The role of renal counterbalance in obstruction has been recognized but only investigated to a limited degree. A possible mechanism for these observations is through neural regulation of renin expression (Chevalier and Thornhill, 1995a). Renal innervation is an important regulator of vascularity and perfusion and is associated with expression of renin in the kidney (el-Dahr et al, 1991). Sympathetic denervation, both mechanical and chemical, has been shown to reduce the expected increase in renin expression in the setting of obstruction (Chevalier and Thornhill, 1995b
Clinical Context
Clinical Presentation of Obstruction
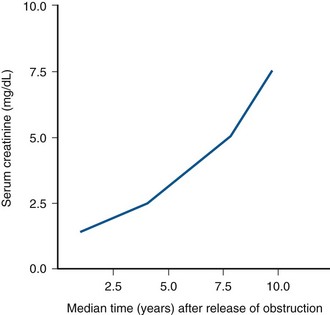
Progressive Renal Dysfunction
Definition of Obstruction
Patterns of Congenital Obstructive Nephropathy
General Observations
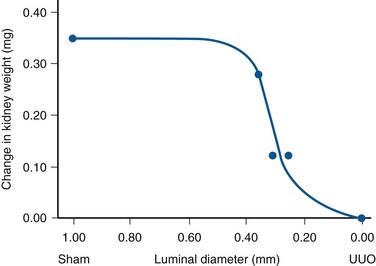
Patterns of Effect
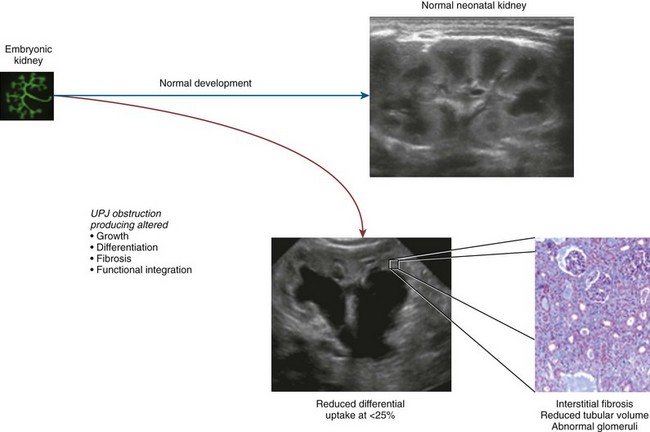
Growth and Differentiation
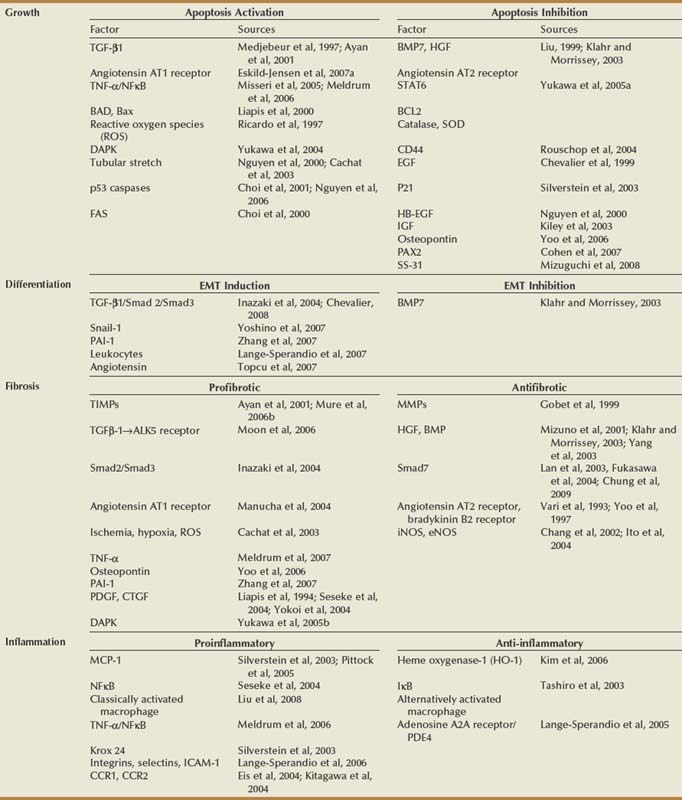
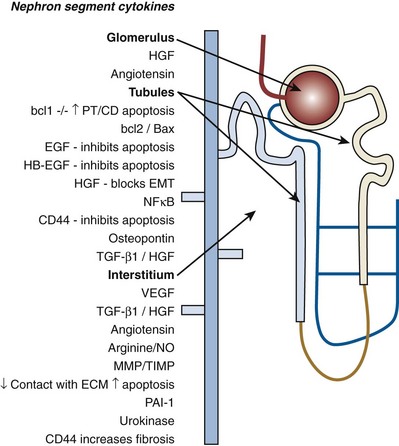
Growth
Growth Regulation
Apoptosis Regulation
Differentiation
Induction Process
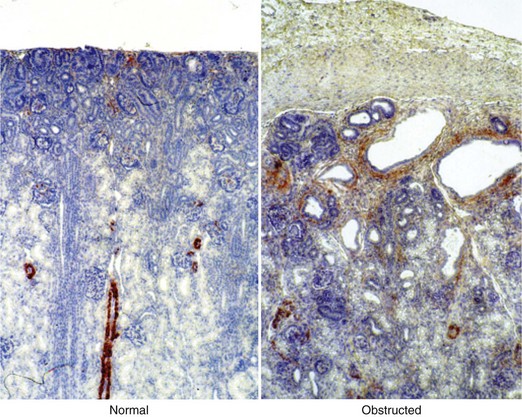
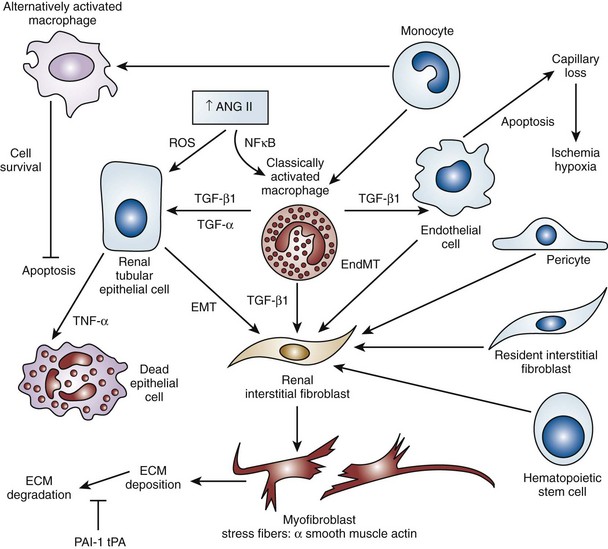
Fibrosis
Evidence and Patterns
Functional Integration
Vascular Development
Neural Development
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Congenital Urinary Obstruction: Pathophysiology and Clinical Evaluation
• Impairment of renal functional development should be considered a determinant of obstruction that warrants intervention.