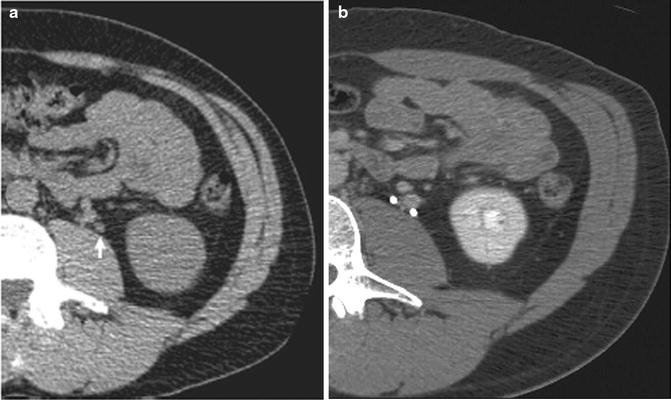
Fig. 34.1
Leadbetter-Politano intravesical ureteral reimplantation. From Kayler L, Kang, Molmenti E et al. Kidney transplant ureteroneocystostomy techniques and complications: review of the literature. Transplant Proc 2010;42:1413–1420. Reprinted with permission from Elsevier Inc
An extravesical approach was described by Lich and Gregoir (Fig. 34.2) in 1961 [2, 3]. With this simplified technique, a single small cystotomy is created at the bladder dome and the distal ureter is anastomosed to the bladder mucosa. An antireflux mechanism is created by closure of the detrusor muscular fibers over the distal ureter. The extravesical approach has emerged as the preferred method in many centers worldwide as it avoids the need for a second cystotomy, requires less ureteral length, is generally associated with less postoperative complications, is faster, and technically less challenging to perform [4, 5].
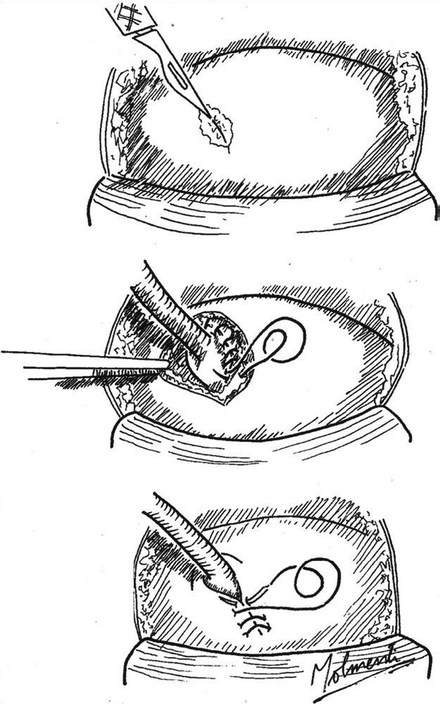

Fig. 34.2
Lich–Gregoir extravesical ureteral reimplantation. From Kayler L, Kang, Molmenti E et al. Kidney transplant ureteroneocystostomy techniques and complications: review of the literature. Transplant Proc 2010;42:1413–1420, reprinted with permission from Elsevier Inc
The location of the ureteric neo-orifice at the bladder dome after the extravesical technique may make subsequent transurethral ureteric access difficult or impossible in the event of a postoperative complication. Advocates of the intravesical technique have proposed that the higher rate of postoperative complications with this technique is offset by easier endoscopic access to the ureter due to its location in proximity to the native ureteric orifice on the posterolateral bladder wall. Tillou and associates [4] evaluated the success of endoscopic management for each of the two common ureteroneocystostomy techniques in a retrospective series of 43 patients requiring endoscopic management of complications following transplantation. Successful endoscopic management was achieved in 75% of those who underwent an intravesical technique as compared with 53% for those who had a Lich–Gregoir extravesical reimplantation. Although ureteric access is simplified by the intravesical technique, one must be mindful that this technique is generally associated with a higher rate of complications, particularly ureterovesical stenosis. A study comparing complications related to the two techniques demonstrated that the rate of urological complications was 9.4% and 3.4% for the intravesical and extravesical approach, respectively [5].
Ureteral obstruction, whether intrinsic or extrinsic occurs in up to 10% of renal transplant recipients [6, 7]. In a meta-analysis considering the role of routine stenting following transplantation, Wilson and colleagues [8] found stent placement to be associated with reduced rates of both urinary obstruction and leakage. The beneficial effect of routine stenting needs to be balanced against a higher rate of urinary tract infection in this group. Additionally, although stents are generally well tolerated, longer stents (≥20 cm) for longer periods (>6 weeks) may be associated with higher rates of encrustation and migration [8]. Particularly in the immediate postoperative period, complications may compromise graft function and result in significant clinical deterioration for patients who are immunosuppressed and have preexisting renal and cardiovascular comorbidities. When recognized and managed in timely manner, such complications are associated with a negligible effect on graft or patient survival [9].
While there remains an important role for open surgical exploration in select cases, improvements in endoscopic equipment and ancillary devices have lead to excellent outcomes with minimally invasive endoscopic techniques. All such techniques require ureteric access, either retrograde or antegrade. Percutaneous antegrade techniques are now generally regarded as the preferred first line approach to ureteric pathology following transplant [10]. With appropriate imaging facilities and interventional radiology expertise, percutaneous allograft nephrostomy can be achieved with minimal morbidity [11]. Advocates for an antegrade approach argue that compared with retrograde transurethral ureteric access, the antegrade technique affords the opportunity to perform a nephrostogram to evaluate the entire renal collecting system and ureter. The location, length, and nature of ureteric obstruction can be evaluated, and intervention in the form of dilatation, endoureterotomy, and/or stent placement can occur. Following such intervention, a percutaneous tube may be left in place to maximize drainage and to allow confirmation that the obstruction has resolved prior to tube removal.
Although the published experience with the retrograde approach is less, Gerrard and colleagues suggested that in many cases, a percutaneous approach may be avoided by an attempt at transurethral access [12]. The likelihood of success with the retrograde technique depends on the location of the ureteroneocystostomy. When an extravesical (Lich–Gregoir) ureteroneocystostomy is performed, the neo-orifice is located at the bladder dome. In recognition of the difficulties accessing the orifice in this location, several investigators have described techniques for ureteric access [12]. There should be a low threshold to utilize flexible instrumentation. A guidewire should be passed if possible to the level of the renal pelvis. Because of the acute angle between the bladder neck and neo-orifice on the bladder dome this wire should be exchanged for an Amplatz extra-stiff wire prior to contemplated intervention to minimize the likelihood of losing the wire and access to the ureter. There remains a lack of data with regard to the role for a ureteral access sheath in this context. Although it is not our practice to utilize a sheath in this scenario, a short sheath placed carefully under fluoroscopic guidance may in some cases facilitate repeated passage of flexible instruments, reduce intra-pelvic pressure, and maximize irrigant flow. In a series of 52 patients with posttransplant ureteric obstruction over a period of 12 years, Gerrard et al. [12] were able to successfully access the ureter and place a ureteric stent in a retrograde fashion in 54% of cases utilizing the above technique. The authors proposed that the retrograde approach may be an appropriate first line management option and a means of avoiding the morbidity associated with percutaneous antegrade renal access.
The usual technique of passing a ureteral stent under direct vision over a wire through the working channel of a rigid cystoscope can be accomplished only when the neo-orifice is visible with rigid instrumentation. When flexible cystoscopy is required, alternative techniques utilizing fluoroscopy have been described [13]. In particular, we advocate the use of an 8/10-Fr coaxial dilator (Fig. 34.3). This device may be passed under fluoroscopy over a guidewire until the 10-Fr outer component is located within the distal ureter. The 8 Fr component is then removed, and a stent may be advanced through the 10-Fr sheath which acts as a buttress to augment subsequent stent passage. A stent pusher with a radiopaque marker is used to position the distal end of the stent. The 10-Fr sheath is then removed along with the wire and the distal coil of the stent should be located within the bladder. The ureteric stent should be removed with a flexible cystoscope at 1 week postoperatively unless a longer duration of stent placement is indicated in scenarios such as ongoing obstruction or intraoperative ureteric perforation. Where ureteric access has been achieved to evaluate and treat obstruction, it is prudent to reevaluate the patient with renal ultrasonography after stent removal to confirm that there is no evidence of persistent hydroureteronephrosis.


Fig. 34.3
8/10 Fr coaxial dilator
Although the superficial location of the transplant kidney makes it amenable to imaging with ultrasound, antegrade access to the transplant kidney is complicated by anomalies in orientation, vascular supply, and location of the graft. Additionally, due to challenges in retrograde access to the ureter and intolerance of intravenous contrast due to the potential effect on graft function, it may not be possible in some cases to opacify the collecting system with contrast. The abnormal orientation of the allograft collecting system complicates puncture in several ways [14]. As compared with the usual approach in a native kidney where access is achieved via a posteriorly oriented calyx, access in the transplant kidney is usually via an anterior calyx. It may be challenging to enter the collecting system at the tip of the calyx and puncture of the infundibulum or renal pelvis may occur. Both of these factors increase the risk of hemorrhagic complications such as pseudoaneurysm and AV fistula. Peri-graft fibrosis may also increase the difficulty of tract dilatation once guidewire access is achieved [15].
With appropriate image guidance in the form of ultrasound or computerized tomography (CT), the interventional radiologist may avert many of the abovementioned factors after making a thorough assessment of the paranephric anatomy prior to tract placement. Additionally, particularly for unstable patients, image-guided percutaneous renal access has the advantage of being able to be performed under local or neurolept anesthesia in most cases.
In exposing the patient to the risks of percutaneous access, one must ensure that the nephrostomy provides appropriate access to the collecting system and ureter. Acute angulation, particularly between the tract and the ureter may make subsequent passage of wires, stents, angiographic catheters, and dilating balloons challenging [16].
Figure 34.4 demonstrates successful antegrade stent placement for treatment of a proximal ureteric stricture in a transplant kidney. After ultrasound-guided access was achieved, a nephrostogram was performed to define the location and length of the ureteric obstruction. A 0.035 in. guidewire was passed through the level of obstruction and a stent placed in satisfactory position with distal and proximal coils in the urinary bladder and transplant renal pelvis, respectively.
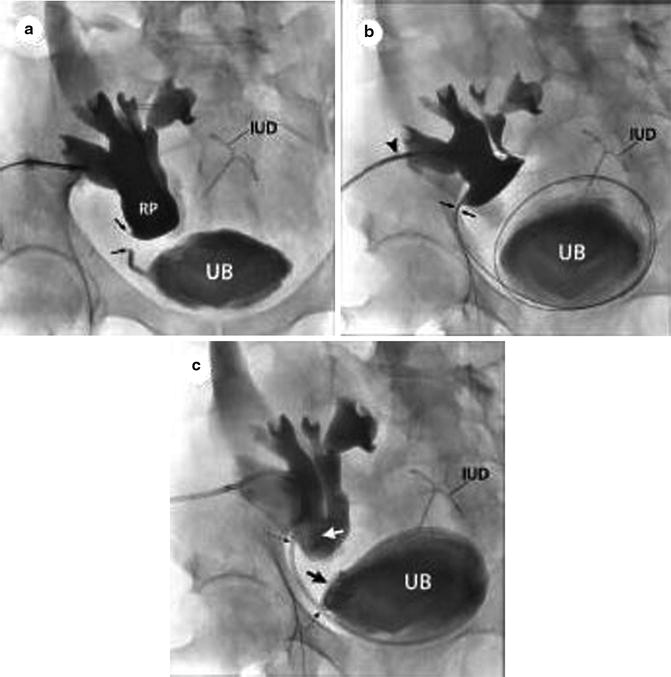

Fig. 34.4
Placement of a nephroureteral stent in a transplant kidney. Ultrasound guidance was used to obtain access (a). A guidewire has been placed across the proximal ureteric stricture (b) (arrow) with subsequent placement of a ureteric stent in satisfactory position (c). From [16], reprinted with permission from W.B./Saunders Co
In summary, access to the ureter in the transplant patient offers unique challenges. When performed correctly, both the retrograde and antegrade approaches are viable, safe options in this patient cohort.
Urinary Tract Duplication
Duplication of the urinary tract is a common congenital abnormality with a reported incidence of 0.8% in autopsy series [17]. Ureteral duplication may be partial or complete. In a bifid system, the collecting systems of the upper and lower moieties join at the uretero-pelvic junction (UPJ). When the confluence of the ureters occurs below the UPJ and above the bladder, a partial or incomplete duplication results. In the case of complete duplication, two ureters enter the bladder at separate locations. As compared with partial duplication, where the ureteric orifice is usually orthotopic, the rates of pathology associated with complete duplication are much higher due to associated vesico-ureteric reflux, ectopia, and ureterocele formation [18]. The right and left kidneys are affected equally. There does appear to be a genetic component, with a 12.5% incidence of duplication in parents or siblings of an affected child [19].
A detailed understanding of the embryologic development of this condition forms the basis of successful identification and management of subsequent ureteric pathology. During normal development, the ureteric bud arises from the mesonephric (Wollfian) duct at 4 weeks and extends cranially to fuse with the metanephric blastema. Complete duplication occurs when two separate ureteral buds arise from the mesonephric duct. The upper and lower pole ureteric buds fuse caudally with the urogenital sinus (UGS) at different time points, leading to the classic configuration of the ureteric orifices as described by Carl Weigert and Robert Meyer [20, 21]. The lower ureteric bud associated with the lower moiety is the first to incorporate into the UGS and hence migrates more laterally on the trigone. The Weigert–Meyer rule states that the upper pole ureter is medial and caudal in relation to the lateral and cranially positioned lower pole ureter. By convention, the bud which drains the lower pole generally represents the normal bud. One must be cognizant of the potential for the upper pole ureter to insert ectopically. In females, an ectopic ureter may enter anywhere from the bladder neck to the perineum and may also insert into the vagina, uterus, or rectum. In males, the ectopic insertion occurs above the external sphincter and involves the Wolffian derived structures such as the seminal vesicles, ejaculatory ducts, and vas deferens.
The likelihood of successful endourologic management in the context of ureteric duplication hinges on preoperative delineation of the anomalous anatomy with appropriate imaging. Prior to the widespread availability and use of noncontrast CT scanning, the urinary system was routinely evaluated with excretory urography (IVP). This technique effectively identified cases of asymptomatic duplication. CT offers many benefits over IVP including speed, accuracy of stone identification and allows detailed assessment of surrounding structures [22]. It also obviates the need for administration of intravenous contrast. While this may be beneficial in the vast majority of cases, Eisner and associates [23] demonstrated that axial noncontrast CT was only 59% sensitive for detecting ureteral duplication when interpreted by specialized genitourinary radiologists. When contrast was administered and a delayed excretory phase was performed, the sensitivity increased to 96% (Fig. 34.5). This study reinforces the importance of carefully evaluating a noncontrast scan. Where doubt exists, further assessment of the collecting system with contrast-enhanced imaging is recommended to identify the location of the ureteric orifice in a completely duplicated system and to demonstrate the branch point of a partially duplicated ureter [23]. Failure to appreciate a partial or complete duplication preoperatively and instrumentation of the wrong ureter may lead to the erroneous conclusion that a stone has passed or is inaccessible with potential clinical consequences.