Colorectal Cancer: Screening and Surveillance
Bernard Levin
Colorectal cancer is the second most common cancer and the second most common cause of cancer death in the United States for both men and women (1). In women, it is responsible for approximately 11% of all new cancer cases and 11% of cancer deaths; in men, it is 10% for both measures. In 2006, the American Cancer Society estimated that approximately 153,000 new cases were diagnosed and that the disease had claimed 53,000 lives (1).
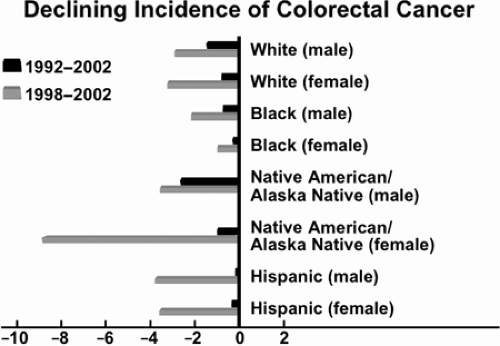
More than any other characteristic, age and its advancement signal risk, as demonstrated by the data in Table 39.1 (2). Colorectal cancer incidence and mortality rates have been declining since approximately 1985 (Fig. 39.1), but overall incidence and mortality continue to be higher in African Americans than in their white counterparts. Furthermore, the declines in incidence found for the overall population are not found to the same degree in the African American population (Fig. 39.2). Declines in the overall U. S. mortality rate have been attributed to greater surveillance of those at risk, improved diagnostic techniques, broadening use of effective adjuvant therapy, and, recently, better therapy for metastatic disease (2). Statistically significant gains have been made in 5-year survival rates for colon cancer and rectal cancer since 1992 for all races, according to the 1975 to 2003 statistics of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program (2).
Internationally, colorectal cancer incidence is highest in Japan, Australia and New Zealand, western Europe, and northern Europe, which indicates its more common occurrence in developed nations (3). Survival estimates are better on average than those for cancers at less common sites, and they are highest in North America (65%), Western Europe (54%), eastern Europe (34%), and India (30%) (3). The rising incidence in Asian countries, of which Japan is a notable example (4), is attributed to adoption of Western lifestyles, especially diet, although other unknown factors cannot yet be excluded.
In the United States, the declining overall incidence and mortality rates, as well as the upward trend in colorectal cancer 5-year survival rates, are positive signs. Educational campaigns through the media with the endorsement of government and professional organizations may further improve early detection rates and expand gains for all groups (5). Advances in molecular genetics have given families at high risk of colorectal cancer powerful lifesaving tools to enhance screening, early detection, and prevention of cancer (6,7).
Widespread screening for colorectal neoplasia over the next decade could lead to a 50% reduction in annual colorectal cancer death rate (8,9). The following sections examine the screening process and recommended colorectal screening tests; discuss their appropriate use among populations of average, moderate, and high risk; and consider current screening participation and how it could be improved.
Screening
Screening for colorectal cancer has the goal of reducing morbidity and mortality from colorectal cancer. Risk categories are (a) average-risk individuals are those age 50 years and older in a Western or “westernizing” country, and (b) higher-risk individuals with a family history or a personal history of colorectal neoplasia or chronic inflammatory bowel disease. These latter individuals require more intensive surveillance. The screening process should embody the characteristics shown in Table 39.2.
The long-recognized standard examinations for colorectal cancer screening have included digital rectal examination, fecal occult blood test (FOBT), sigmoidoscopy, double-contrast barium enema, and colonoscopy. Of these five, digital rectal examination is generally no longer recommended because alone it has not proven effective in colorectal cancer screening. The 7- to 8-cm reach of the examining finger could detect at best only 10% of colorectal cancers. Nonetheless, digital rectal examination remains a part of sigmoidoscopy, colonoscopy, and barium enema examinations and may be considered a component of comprehensive preventive health care (10).
Fecal Occult Blood Testing
Types of Fecal Occult Blood Tests: Guaiac and Immunochemical Tests
FOBTs are based on two principal techniques: chemical tests and immunochemical tests. The major features of these tests are described in Table 39.3. Key usage and performance issues related to different types of FOBTs are described in Tables 39.4 and 39.5.
Effectiveness
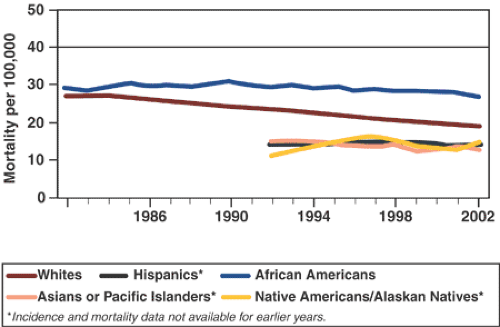 FIGURE 39.1. U.S. colorectal cancer mortality. Source: Adapted from Surveillance, Epidemiology, and End Results (SEER) Program and NCHS, 2006. (See also color Figure 39.1) |
FOBT is the most rigorously and extensively studied method used to detect colorectal cancer in asymptomatic persons (11). Bleeding occurs from cancer and large adenomas but probably not from small adenomas; hence, sensitivity of the test for detection of these lesions is limited. Researchers have demonstrated that FOBTs, whether performed annually or biennially, reduce mortality from colorectal cancer (12,13). The tests used are the six sample-based home FOBTs and not the digital office test. The latter has been shown to be significantly inferior (14).
Annual screening, using a guaiac-based method (Hemoccult) according to the Minnesota Trial, reduced mortality by 33% (15). In a meta-analysis of mortality results from randomized controlled trials, Hewitson et al. (12) found the reduction to be 16% overall (relative risk [RR], 0.84; confidence interval [CI], 0.77–0.92) (Fig. 39.3) and 23% when adjusted for screening attendance (11).
Annual screening, using a guaiac-based method (Hemoccult) according to the Minnesota Trial, reduced mortality by 33% (15). In a meta-analysis of mortality results from randomized controlled trials, Hewitson et al. (12) found the reduction to be 16% overall (relative risk [RR], 0.84; confidence interval [CI], 0.77–0.92) (Fig. 39.3) and 23% when adjusted for screening attendance (11).
Table 39.1 Colorectal cancer incidence and mortality rate for selected groups | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The success of FOBT in reducing colorectal cancer mortality is enhanced by other benefits, including a lowering of incidence due to removal of adenomatous polyps after detection (15) and a potential reduction in surgical intervention because of earlier detection of disease. The FOBT is both effective and cost efficient; has a long record of testing in the United States and abroad; and has been recommended by government, professional organizations, and patient advocacy groups.
The major shortcoming of FOBT—its high false-positive rate—is viewed as something of a virtue by some, inasmuch as more rigorous testing follows the initial false-positive findings, and true cases are subsequently detected (Table 39.3 provides sensitivity and specificity measures) (11,16). Others find intolerable the unnecessary risks and stresses that patients wrongly identified must endure with colonoscopy (12). In contrast, a negative FOBT could falsely reassure patients and lead to delayed response to the development of colorectal symptoms (17).
There has been considerable recent interest in the use of fecal immunochemical tests (FITs). FITs use antibodies specific for human globin and thus are not affected by dietary hemoglobin or peroxidase. A variety of stool sampling methods have been developed, including the use of a wooden spatula,
probe, or brush. Using a predetermined standard threshold of hemoglobin to indicate a positive test, laboratory development of a quantitative method is preferable to a qualitative result. The test output is a continuous variable that offers the option of adjusting the threshold to suit the objective. The U.S. Food and Drug Administration approved the use of an FIT in 2001. Subsequently, the American Cancer Society recommended using FITs instead of guaiac-based FOBT because of ease of use and better sensitivity and specificity (18). Smith et al. compared a sensitive guaiac FOBT (Hemoccult II Sensa, Beckman-Coulter, Fullerton, CA) with a brush-sampling FIT (Insure, Enterix, Inc, Edison, NJ) in both a screening cohort (n = 2,351) and a symptomatic diagnostic group (n = 161). Combining results for both cohorts, the FIT returned a true-positive result notably more often in cancer (n = 24, 87.5% vs. 54.2%) or significant adenomas (n = 61, 42.6% vs. 23.0%). In the screening cohort, the false-positive rate for any neoplasia was marginally higher than the guaiac FOBT (3.4% vs. 2.5%, 95% CI of difference), whereas positive predictive values were 41.9% and 40.4%, respectively (19). Levi et al. (19) compared colonoscopy with hemoglobin content of three bowel movements in 1,000 patients, some asymptomatic but at increased risk for colorectal neoplasia and some symptomatic, using an FIT (OC-MICRO, Eiken Chemical Co., Tokyo, Japan). Colonoscopy identified clinically significant neoplasia in 91 patients (cancer in 17 patients and advanced adenomas in 74 patients). Using three FITs and a hemoglobin threshold of 75 mg/mL, sensitivity and specificity were 94.1% (95% CI, 82.9%–100%) and 87.5% (85.4%–89.6%), respectively, for cancer and 67% (CI, 57.4%–76.7%) and 91.4% (CI, 89.6%–93.2%), respectively, for any clinically significant neoplasia (20). These data may not be completely applicable to an average-risk screening population, but the methodology may well be suited to enhance the effectiveness of our current strategies (20).
probe, or brush. Using a predetermined standard threshold of hemoglobin to indicate a positive test, laboratory development of a quantitative method is preferable to a qualitative result. The test output is a continuous variable that offers the option of adjusting the threshold to suit the objective. The U.S. Food and Drug Administration approved the use of an FIT in 2001. Subsequently, the American Cancer Society recommended using FITs instead of guaiac-based FOBT because of ease of use and better sensitivity and specificity (18). Smith et al. compared a sensitive guaiac FOBT (Hemoccult II Sensa, Beckman-Coulter, Fullerton, CA) with a brush-sampling FIT (Insure, Enterix, Inc, Edison, NJ) in both a screening cohort (n = 2,351) and a symptomatic diagnostic group (n = 161). Combining results for both cohorts, the FIT returned a true-positive result notably more often in cancer (n = 24, 87.5% vs. 54.2%) or significant adenomas (n = 61, 42.6% vs. 23.0%). In the screening cohort, the false-positive rate for any neoplasia was marginally higher than the guaiac FOBT (3.4% vs. 2.5%, 95% CI of difference), whereas positive predictive values were 41.9% and 40.4%, respectively (19). Levi et al. (19) compared colonoscopy with hemoglobin content of three bowel movements in 1,000 patients, some asymptomatic but at increased risk for colorectal neoplasia and some symptomatic, using an FIT (OC-MICRO, Eiken Chemical Co., Tokyo, Japan). Colonoscopy identified clinically significant neoplasia in 91 patients (cancer in 17 patients and advanced adenomas in 74 patients). Using three FITs and a hemoglobin threshold of 75 mg/mL, sensitivity and specificity were 94.1% (95% CI, 82.9%–100%) and 87.5% (85.4%–89.6%), respectively, for cancer and 67% (CI, 57.4%–76.7%) and 91.4% (CI, 89.6%–93.2%), respectively, for any clinically significant neoplasia (20). These data may not be completely applicable to an average-risk screening population, but the methodology may well be suited to enhance the effectiveness of our current strategies (20).
Table 39.2 Screening process | ||
|---|---|---|
|
Table 39.3 Sensitivity and specificity ranges for four colorectal cancer screening examinations | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Flexible Sigmoidoscopy
Offering greater reliability than FOBT and incorporating visualization and removal of potentially life-threatening adenomatous polyps is flexible sigmoidoscopy (FSIG), although its impact is limited to the left side of the colon and the rectosigmoid. Sensitivity varies from 73.3% for small polyps to 96.7% for cancer and large polyps; specificity ranges from 92% for small polyps to 94% for cancer and large polyps (Table 39.3).
The place of sigmoidoscopy in screening was first secured through case-control studies and through research indicating that removal of adenomatous polyps reduces risk of colorectal cancer (21,22). The investigators found that sigmoidoscopy screening reduced colorectal cancer mortality from disease detectable by the procedure by 70% to 80%. A prospective controlled study published in 1999 found that FSIG (followed on discovery of polyps by colonoscopy with polypectomy) had significantly reduced the incidence of colorectal cancer among those screened (p = 0.02); 10 persons in the control group, but only 2 in the screened group, had colorectal cancer during 13 years of study (RR, 0.2; 95% CI, 0.03–0.95). Interestingly, the overall mortality rate from all causes was found to be higher in the screened group (14%) than in the unscreened group (9%), which prompted the researchers to call for more study of overall mortality in screening (23). The United States, through the National Cancer Institute, and the United Kingdom hope to resolve lingering questions about the appropriate use of FSIG in screening by undertaking prospective studies. The National Cancer Institute’s Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial is seeking to document the mortality reduction achieved by screening for these cancers, including that for FSIG, by conducting randomized controlled studies that encompass almost 150,000 subjects at 74 centers across the United States. A report from the PLCO Trial on the baseline screening examination included 64,658 subjects (83.5% of those randomized). The yields per 1,000 screened were as follows: for colorectal cancer, 1.1 to 2.5 in women and 2.4 to 5.6 in men; for advanced adenoma, 18.0 to 30.4 in women and 36.1 to 49.1 in men; and for colorectal cancer or any adenoma, 50.6 to 79.6 in women and 101.9 to 128.6 in men. Approximately, 77% (130/169) of colorectal adenocarcinomas were stage I or II at diagnosis (24).
Table 39.4 Usage and Performance Issues for Different Types of Fecal Occult Blood Tests (FOBTs) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
In the United Kingdom, researchers are evaluating effects of a single FSIG examination in 120,000 participants over
15 years (25,26). In the United Kingdom, the baseline findings of a multicenter trial showed that of approximately 40,000 patients screened, distal adenomas were detected in 12%, and distal cancers were detected in 0.3% (27).
15 years (25,26). In the United Kingdom, the baseline findings of a multicenter trial showed that of approximately 40,000 patients screened, distal adenomas were detected in 12%, and distal cancers were detected in 0.3% (27).
Table 39.5 Comparison of colorectal cancer screening recommendations for average-risk adults 50 years or older | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree