Colorectal Cancer: Pathology
Thomas C. Smyrk
Multiple molecular pathways may contribute to colon carcinogenesis, and colorectal carcinoma may be a more heterogeneous disease than is generally acknowledged. Nevertheless, one morphologic continuum—the dysplasia-carcinoma sequence—can encompass the histologic range of malignant and premalignant epithelial changes, and it is reasonable to discuss colorectal carcinoma as a single pathological entity. This chapter reviews the known precursor lesions, describes the histology of colorectal carcinoma, and discusses specific features associated with one molecular subtype (i.e., microsatellite instability-high [MSI-H] carcinoma).
Precursor Lesions
Aberrant crypt foci (ACF) are the earliest grossly visible precursors to adenocarcinoma (1). They can be identified in situ with the aid of magnifying endoscopy and methylene blue staining (2). Based on the combination of endoscopic appearance and histology, three types of ACF have been characterized: (a) nondysplastic, nonhyperplastic; (b) nondysplastic, hyperplastic; and (c) dysplastic. The first have round or oval lumens when viewed endoscopically; hyperplastic ACF have slitlike lumens and dysplastic ACF have compressed or indistinct lumens with a thickened epithelial lining. The histologic appearance of ACF parallels the gross changes, ranging from normal to hyperplastic to dysplastic. The number of dysplastic ACF progressively increases from normal controls to adenoma patients to cancer patients, suggesting that dysplastic ACF are precursors to adenoma (2). The most important application for ACF at this time is as an end point in chemoprevention studies (3).
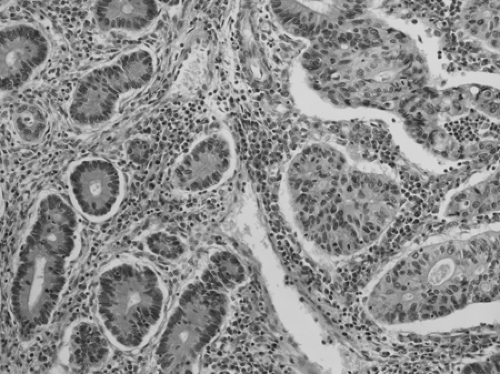
Adenomas are grossly visible collections of dysplastic crypts. Macroscopically, adenomas can be elevated, flat, or depressed. Elevated adenomas range from pedunculated polyps on a stalk to sessile lesions. Flat or depressed adenomas can be recognized by mucosal reddening or changes in texture. Whatever the gross morphology, adenoma is defined by the presence of intraepithelial neoplasia, and recognizable by hypercellularity, enlarged hyperchromatic nuclei, and varying degrees of nuclear stratification (Fig. 41.1). The intraepithelial neoplasia can be classified as low-grade or high-grade, depending on the degree of architectural complexity and nuclear stratification, pleomorphism, and loss of polarity. The term tubular adenoma is applied when dysplastic glandular structures are present on at least 80% of the luminal surface. If more than 80% of the surface is covered by villiform-to-ridgelike structures, the lesion is termed a villous adenoma. Tubulovillous adenomas have a mixture of tubular and villous architecture. There is a general relationship between size, villosity, and the presence of high-grade dysplasia and invasive carcinoma. The frequency of high-grade dysplasia increases with adenoma size and is highest in villous adenomas (4).
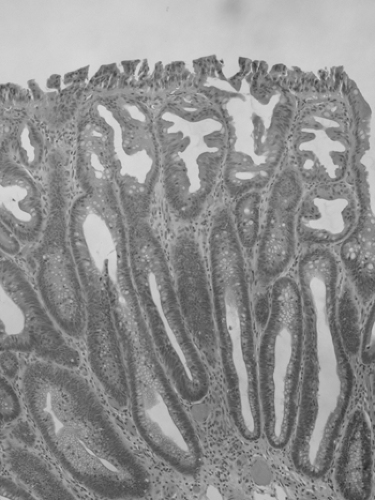
Serrated adenoma (SA) has serrated crypts similar to those seen in hyperplastic polyp, but the crypts are lined by dysplastic epithelium (5) (Fig. 41.2). By convention, the entire lesion should have this appearance in order to be classified as SA. Once believed to be a variant of adenoma, SA is now placed in the general category of serrated polyps (Table 41.1) (6).
Sessile serrated adenoma (SSA) resembles hyperplastic polyp in that it contains serrated crypts, but there are subtle architectural differences (7). Dilation and lateral branching at the crypt base are the easiest to recognize; there is also abnormal maturation in the crypt base, serration at the crypt base, small foci of nuclear crowding and pseudostratification in the crypt walls and in the surface epithelium, and focal eosinophilic change in epithelial cytoplasm (Fig. 41.3). The use of the term “SSA” has been criticized because the lesion generally contains no overt cytologic dysplasia. Other proposed terms include sessile serrated polyp and sessile serrated lesion, but the defenders of SSA argue that cytologic dysplasia is not required for a diagnosis of adenoma in other sites (hepatic adenoma, adrenal cortical adenoma) and that the lesion does have architectural dysplasia. When cytologic dysplasia does develop in SSA, the dysplastic focus often has loss of expression for the mismatch repair protein MLH1 (Fig. 41.4), and when invasive carcinoma develops, it is often MSI-H. In fact, SSA may be the precursor lesion for most or all MSI-H colorectal carcinoma (6).
Colorectal Carcinoma
Macroscopic Features
The macroscopic features of colorectal carcinoma depend in part on the extent of disease progression, but gross tumor configuration can be broadly categorized as exophytic or flat. Flat cancers may be ulcerative or infiltrative. Right-sided tumors tend to form exophytic masses more often than left-sided ones, and infiltrative growth is typical of signet ring cell carcinoma. Exophytic growth has been associated with lower stage (8) and lower risk for hematogenous metastasis (9). In 1939, Grinnell
reported 83% survival for patients with tumors projecting into the bowel lumen, compared to 45% for those with tumors classified as intermediate and 38% for those with infiltrative tumors (10). An exophytic growth pattern was identified as a significant favorable prognostic feature in more recent studies as well (11,12). Whether exophytic or infiltrative, tumors that encircle more than three-fourths of the bowel circumference are associated with adverse outcome (13), and obstruction is a clinical marker of poor prognosis (14,15,16,17). In practice, overlapping growth patterns and variability in observer interpretation of macroscopic configuration limit the usefulness of this parameter. A consensus statement from the College of American Pathology (CAP), citing these difficulties, made no recommendation as to reporting guidelines for tumor configuration (18). Still, studies continue to report better outcomes for polypoid carcinoma (19). A reasonable suggestion is that pathologists attempt to characterize configuration as exophytic (polypoid), ulcerative, or infiltrative, and, for annular tumors, estimate the percentage of bowel circumference involved.
reported 83% survival for patients with tumors projecting into the bowel lumen, compared to 45% for those with tumors classified as intermediate and 38% for those with infiltrative tumors (10). An exophytic growth pattern was identified as a significant favorable prognostic feature in more recent studies as well (11,12). Whether exophytic or infiltrative, tumors that encircle more than three-fourths of the bowel circumference are associated with adverse outcome (13), and obstruction is a clinical marker of poor prognosis (14,15,16,17). In practice, overlapping growth patterns and variability in observer interpretation of macroscopic configuration limit the usefulness of this parameter. A consensus statement from the College of American Pathology (CAP), citing these difficulties, made no recommendation as to reporting guidelines for tumor configuration (18). Still, studies continue to report better outcomes for polypoid carcinoma (19). A reasonable suggestion is that pathologists attempt to characterize configuration as exophytic (polypoid), ulcerative, or infiltrative, and, for annular tumors, estimate the percentage of bowel circumference involved.
Table 41.1 Serrated polyps of the colorectum | |
|---|---|
|
Sessile serrated adenomas tend to arise in the right colon. They can become large; most are >0.5 cm, and plaques 2 to 3 cm in diameter are not unusual. Current recommendations are preliminary but include complete endoscopic removal or regular endoscopic surveillance with biopsy if the lesion cannot be removed. If overt dysplasia develops in an SSA, then resection should be considered. Repeat endoscopy at an interval similar to that for patients with adenoma is recommended even for patients with a completely removed SSA (6).
Histologic Subtypes
The current World Health Organization (WHO) classification recognizes the following histologic categories:
adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, squamous and adenosquamous carcinoma, small cell carcinoma, medullary carcinoma, and undifferentiated carcinoma (20).
adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, squamous and adenosquamous carcinoma, small cell carcinoma, medullary carcinoma, and undifferentiated carcinoma (20).
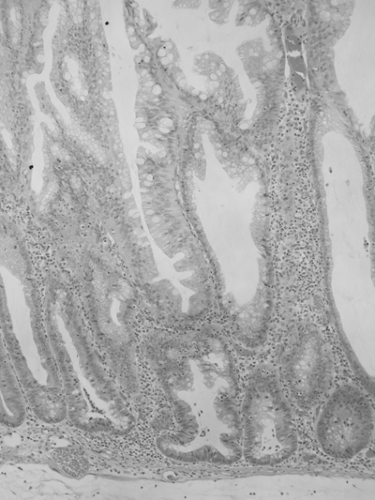 FIGURE 41.3. Sessile serrated adenoma. Serrated crypts with abnormal architecture but without overt dysplasia. Lateral extension at the crypt base is the most helpful diagnostic feature. |
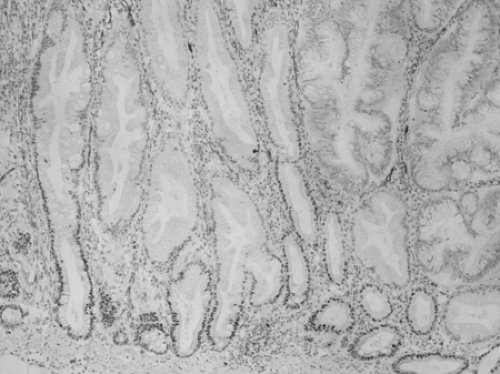 FIGURE 41.4. Transition to dysplasia in a sessile serrated adenoma, accompanied by loss of nuclear MLH1 expression. Immunohistochemistry for MLH1, counterstained by hematoxylin. |
Most colorectal carcinomas (85%–90%) are adenocarcinomas without special morphologic features. They are gland-forming tumors with variability in gland size and shape. The lining cells are tall and columnar in better-differentiated tumors, becoming more cuboidal with decreasing degrees of differentiation. There is a corresponding change in nuclear morphology, ranging from oval and fairly regular to round and pleomorphic. The gland lumens are often filled with debris, producing the “dirty necrosis” that is a helpful diagnostic clue in both primary and metastatic lesions.
The differential diagnosis of colorectal cancer includes other primary colon tumors (carcinoid, malignant lymphoma, epithelioid variants of gastrointestinal stromal tumor), tumors invading the colon by direct extension (prostate, endometrium, ovary), and metastases from other organs. The presence of gland-forming areas, confirmed by a stain for mucin if necessary, is usually sufficient to eliminate carcinoid, lymphoma, and stromal tumor from consideration, but the undifferentiated medullary subtype of colon cancer can mimic all three of these. Clinical information is, of course, helpful in recognizing noncolonic tumors involving the colon by direct extension or metastasis, but the diagnosis can be suspected when mural growth predominates over mucosal growth.
Although immunohistochemistry is rarely necessary to confirm the diagnosis of colorectal carcinoma, there is a characteristic immunohistochemical profile. More than 95% of colorectal cancers are positive for CDX2 protein, produced by a homeobox gene encoding an intestine-specific transcription factor (21). The protein is not specific for colorectal carcinoma, being immunodetected in 25% to 70% of adenocarcinomas from elsewhere in the gut and in most neuroendocrine tumors of gastrointestinal origin. Most colorectal cancers express CK20 and are negative for CK7, but one should be careful about requiring that profile for the diagnosis; poorly differentiated or undifferentiated carcinoma can be negative for both CK20 and CK7 (5% of total cases), and it is not unusual to see some immunopositivity for CK7, particularly in rectal cancers (22).
 FIGURE 41.5. Mucinous carcinoma, diagnosed when extracellular mucin accounts for >50% of tumor volume. |
Mucinous adenocarcinomas account for 10% of colorectal cancers. A tumor is classified as mucinous if more than 50% of its volume consists of mucin (Fig. 41.5). The prognostic significance of mucinous histology has been debated. Of seven studies published in the 1990s, four found no prognostic significance associated with this histology (16,23,24,25), two found adverse effect by univariate analysis but not multivariate analysis (26,27), and one found mucinous histology to be predictive of recurrence in patients younger than 45 years (28). The CAP consensus is that mucinous differentiation is not proven to be a statistically significant factor independent of histologic grade (18). Substantial mucin production is a feature of cancers with MSI; mucinous carcinoma is about twice as likely as usual adenocarcinoma to be MSI-H (30% vs. 15%) (29). MSI-H mucinous adenocarcinoma has a better outcome than microsatellite stable mucinous adenocarcinoma (29), so studies that do not subclassify mucinous adenocarcinoma by MSI status may be skewed, depending on the prevalence of MSI-H cancers in the study set.
Signet ring cell carcinomas comprise approximately 2% of colorectal cancers. The characteristic cell has an intracytoplasmic mucin-containing vacuole that pushes the nucleus to the periphery (Fig. 41.6). More than 50% of the tumor should be made up of signet cells for a diagnosis of signet ring cell
carcinoma (20), although molecular studies demonstrate that tumors with a minor signet cell component are similar to those more abundant signet cells in terms of BRAF mutations, MSI and other molecular markers (30). The tumor is rare enough that signet ring cell histology (and the infiltrative gross tumor configuration that typically accompanies it) should prompt consideration for metastasis from gastric carcinoma or lobular carcinoma of the breast. Most descriptions of primary signet ring cell carcinoma emphasize its poor prognosis (31,32), but the literature is mixed, and a small study by Giacchero et al. (nine cases of signet ring cell carcinoma) found no effect on stage-adjusted survival (33). About 30% of signet ring cell carcinomas are MSI-H, which could confound survival data when that factor is not accounted for. In one study, however, MSI status did not affect outcome within signet ring cell carcinoma patients (34). It has been suggested that 30% of patients with signet ring cell carcinoma have ulcerative colitis (35), so differences in the molecular pathogenesis and natural history of carcinoma in that setting could also confound outcome studies.
carcinoma (20), although molecular studies demonstrate that tumors with a minor signet cell component are similar to those more abundant signet cells in terms of BRAF mutations, MSI and other molecular markers (30). The tumor is rare enough that signet ring cell histology (and the infiltrative gross tumor configuration that typically accompanies it) should prompt consideration for metastasis from gastric carcinoma or lobular carcinoma of the breast. Most descriptions of primary signet ring cell carcinoma emphasize its poor prognosis (31,32), but the literature is mixed, and a small study by Giacchero et al. (nine cases of signet ring cell carcinoma) found no effect on stage-adjusted survival (33). About 30% of signet ring cell carcinomas are MSI-H, which could confound survival data when that factor is not accounted for. In one study, however, MSI status did not affect outcome within signet ring cell carcinoma patients (34). It has been suggested that 30% of patients with signet ring cell carcinoma have ulcerative colitis (35), so differences in the molecular pathogenesis and natural history of carcinoma in that setting could also confound outcome studies.
Squamous cell carcinoma of the colorectum is very rare. Keratin and intercellular bridges must be identified to make the diagnosis, and no glandular areas should be present. Because squamous carcinoma is common in the anal canal, no continuity must exist between the tumor and the anal canal. In addition, no evidence should be seen of primary squamous carcinoma at a site that could be a source of metastasis to the colon. In a 1979 review, Williams et al. found less than 30 reported cases meeting these criteria (36). More recently, a single-institution review of 4,561 colorectal cancers discovered two acceptable cases of squamous carcinoma (37).
Adenosquamous carcinoma is diagnosed when a gland-forming carcinoma has areas of squamous differentiation. No agreement exists regarding the amount of squamous epithelium required for the diagnosis, but the WHO text advises that “there should be more than just small foci of squamous differentiation” (20). Two cases of adenosquamous carcinoma reported by Cerezo et al. behaved aggressively, with distant metastases from the squamous component (38), but no definitive statement is possible about the natural history of this histologic type.
Small cell carcinomas account for <1% of colorectal cancers. The tumor is identical to its counterpart in the lung, forming sheets of malignant cells with round, dark nuclei and little or no visible cytoplasm (Fig. 41.7). Wick argued convincingly for the replacement of the term small cell carcinoma (in any organ) with neuroendocrine carcinoma, grade 3 (39). By any name, this is an aggressive tumor notorious for early hematogenous spread. Of 38 patients with small cell carcinomas of the large intestine reported by the Armed Forces Institute of Pathology, 71% had liver metastases and 64% were dead at 5 months (40). A remarkable feature of small cell carcinoma is the frequent presence of an overlying adenoma, with abrupt transition from the innocuous-appearing adenoma to the aggressive carcinoma (41).
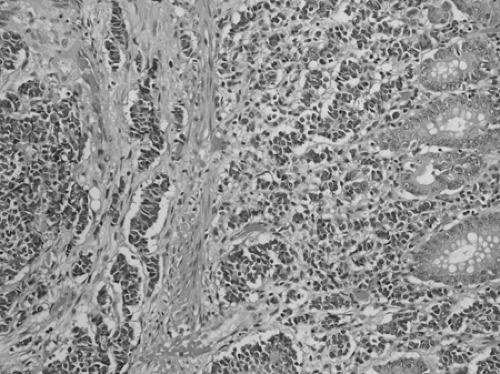 FIGURE 41.7. Small cell carcinoma. In this example, undifferentiated cells appear to drop off the bottom of a tubular adenoma, which is toward the right. |
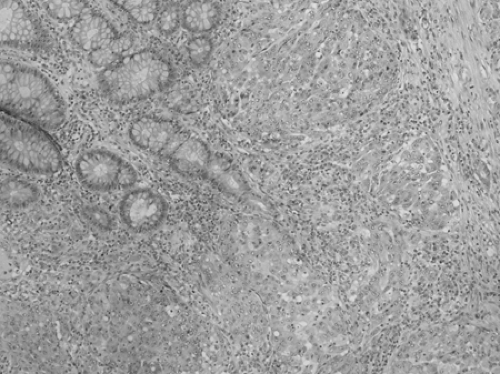 FIGURE 41.8. Medullary carcinoma. Sheets of cells occur, each cell having a round, vesicular nucleus and a prominent nucleus. |
Medullary carcinoma forms a solid pattern of cells with round nuclei and a prominent nucleolus (42,43) (Fig. 41.8). There is little variability in nuclear size and shape. Many lymphocytes are immediately adjacent to tumor cells (“tumor-infiltrating lymphocytes”) (44). Despite its rather ominous appearance, descriptive reports suggest that this tumor is relatively indolent (42,43,45). Medullary carcinoma is almost always MSI-H; this histologic pattern has been described in both the hereditary setting (hereditary nonpolyposis colorectal carcinoma) (46) and in sporadic MSI-H colorectal carcinoma (47).
Undifferentiated carcinoma is defined as a malignant epithelial tumor without glandular structure or other features to indicate differentiation. Small cell carcinoma should not be included in this category, despite its confusing synonym “small cell undifferentiated carcinoma.”
Staging
Tumor stage is the most powerful predictor of prognosis for colorectal cancer. Accurate assignment of stage requires judicious sampling of tumor to assess depth of invasion and a careful search for lymph nodes. In the fresh state, colon cancer is often too friable to be serially sectioned at the intervals necessary to adequately document depth of invasion. Thus, resected bowel with tumor should be opened and fixed in formalin for several hours before dissection. (If fresh tumor is required for ancillary studies, a piece of tissue can usually be removed without disturbing the relevant anatomical relationships.) After fixation, the tumor should be serially sectioned at intervals of 0.5 cm, and samples demonstrating the areas of deepest invasion submitted for microscopic examination.
Lockhart-Mummery, a surgeon at St. Mark’s Hospital in London, proposed the first staging system for rectal cancer (48). The name of Dukes will forever be associated with his
modification of Lockhart-Mummery’s system (49) and the various modifications that followed (50,51,52). Dukes’ system is easy to apply, but the existence of so many competing classifications sometimes produces confusion (53,54). The tumor, node, metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) is the standard and is used here (55).
modification of Lockhart-Mummery’s system (49) and the various modifications that followed (50,51,52). Dukes’ system is easy to apply, but the existence of so many competing classifications sometimes produces confusion (53,54). The tumor, node, metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) is the standard and is used here (55).
Table 41.2 Tumor (T) definitions for the tumor, node, metastasis (TNM) staging system | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The T category, referring to the local extent of untreated primary tumor, is delineated in Table 41.2. When the T status has been evaluated pathologically, it is designated pT. The Tis category sometimes causes confusion. Carcinoma in situ and intraepithelial carcinoma are synonyms; both refer to malignant cells that have not yet escaped the gland basement membrane. (High-grade dysplasia is another synonym for the same situation.) The term intramucosal carcinoma signifies that malignant cells invade lamina propria or extend into muscularis mucosae but do not reach the submucosa. Although the recommendation is that Tis tumors be specified as either intraepithelial or intramucosal carcinoma, neither lesion has potential for lymph node metastasis, and both lesions can be considered cured by complete removal and are thus included in a single grouping. Tumors that penetrate the muscularis mucosae and invade submucosa are pT1.
Evaluation of depth of invasion is usually not difficult in a well-oriented sample of tumor, but differentiation between T2 and T3 can require some care: If even one muscle fiber is present between tumor and perimuscular soft tissue, the tumor is classified as T2. Invasive tumor typically elicits a fibroblastic response (“desmoplastic stroma”) that can obscure the anatomical landmarks. A special stain (trichrome) helps differentiate between muscle and collagen in difficult cases. When there is doubt as to the level of invasion, the general rule is to choose the less advanced category (56).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree